Beef Jerky: Fate of Staphylococcus Aureus in Marinated and Corned Beef During Jerky Manufacture and 2.5 C Storage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

R E S E R V E C
R E S E R V E C U T SPRING DINNER MENU GLATT R E S E R V E C U T R E S E R V E C U T GLATT GLATT ★★★★★ S U S H I & S A S H I M I TUNA 7/9 H O U S E - M A D E & C U R E D M E A T S FLUKE 6/8 SHIMA AJI 7/9 CHARCUTERIE PLATE KAMPACHI 7/9 VEAL SAUSAGE OCEAN TROUT 7/9 HAMACHI 7/9 BEEF SAUSAGE SALMON 7/9 SMOKED LAMB SAUSAGE MADAI 7/9 DUCK PROSCIUTTO IKURA 6/8 WITH ACCOMPANIMENTS TORO MP 32 C U T & H A N D R O L L S BUTCHERS CUT TERIYAKI BEEF JERKY 13 EACH 29 SPICY TUNA ★★★★★ SPICY SALMON AVOCADO CUCUMBER YELLOWTAIL JALAPENO C O L D A P P E T I Z E R S FLUKE SHISHITO PEPPER SALMON AVOCADO CLASSIC ‘WAGYU’ BEEF TARTARE CALIFORNIA SHALLOTS, CORNICHONS, QUAIL YOLK 28 S I G N A T U R E R O L L S YELLOWTAIL CARPACCIO JALAPENO, BLACK CAVIAR, MICRO CILANTRO, TUNA & BLACK TRUFFLE 24 CITRUS PONZU AVOCADO, CRUNCH, TERIYAKI, CILANTRO 24 SEARED MADAI WITH CRISPY SHALLOTS 22 TUNA, AVOCADO, CITRUS TERIYAKI, CILANTRO BIGEYE AHI TUNA TARTARE AVOCADO MOUSSE, SESAME -MEYER LEMON DRESSING YELLOWSTONE 22 24 KANI, YAMAGOBO, CUCUMBER & SWEET BBQ SALMON SALMON TARTARE VOLCANO 22 TORTILLA CHIPS, SCALLIONS, CAYENNE AIOLI SPICY TUNA, AVOCADO, AIOLI SAUCE & TEMPURA 22 SALMON, TUNA & BLACK CAVIAR (NO RICE) 23 RC CRISPY RICE & SPICY TUNA TUNA WRAPPED ATLANTIC SALMON, SCALLIONS CILANTRO SPROUTS & CHIPOTLE AIOLI 21 GARDEN VEGETABLES 21 CARROTS, CUCUMBER, AVOCADO, CARROT GINGER SAUCE CRISPY ALMOND TRIPLE (NO RICE) 23 H O T A P P E T I Z E R S SALMON AND YELLOWTAIL OSHINKO WRAPPED IN TUNA LAMB MERGUEZ SAUSAGE MEDITERRANEAN COUS COUS, ROASTED EGGPLANT, GRILLED CHILEAN SEA BASS 23 BUTTERNUT SQUASH, ZUCCHINI CRISPY CUCUMBER, AVOCADO, TERIYAKI, GARLIC CHIPS 26 M.I.T.B. -

Fat Louie's Menu
Available In Mini / Regular / Whole • Also Available in a Bowl 50% OFF SANDWICHES • PIZZA • SOUTH PHILLY CLASSICS Buy Any AvailableR U In FAT Mini /SANDWICHES Regular / Whole • Also Available in a Bowl DRINKS SANDWICHES • PIZZA • SOUTH PHILLY CLASSICS Sandwich Get Mini 7.99 | Regular 9.99 | Whole 21.99 Fresh Lemonade (24 oz.) 2.99 PAID All IngredientsR U Listed FAT Below Served SANDWICHES ON the Sandwich ECRWSS PRSRT STD PRSRT Bellmawr, NJ Bellmawr, Permit #1927 Permit Fountain Drinks (22DRINKS oz.) 1.99 US POSTAGE 2nd 1/2 Off Mini Regular Whole The Big Fat Louie Bubba-Luck Vitamin Water 2.25 7.69 9.99 21.99 Fresh Lemonade (24 oz.) 2.99 FAT LOUIE’S HOAGIES Cheese steak, whiz, Louie’s fries, fried Chicken tenders, Louie’s fries, fried pickles, 2 Liter Sodas 2.99 mozzarella, time offer Limited Thechopped Big bacon Fat &Louie marinara honey mustard,Bubba-Luck lettuce, tomato & onion Joe’s TeaFountain (20 oz.) 12Drinks Flavors (22 Available oz.) 1.99 2.49 Woodbury 1/2 Off Matty BoomCheese Bots steak, whiz, Louie’s fries, fried The Sac DaddyChicken tenders, Louie’s fries, fried pickles, CBD DrinksVitamin 3 Flavors Water Available 5.99 856-853-1000nd Cheese steak,mozzarella, fried egg &chopped chopped bacon bacon & marinaraPepper Jack honeycheese, mustard, steak, fried lettuce, onions, tomato bacon & onion 2 Liter Sodas The RockMatty Boom Bots & pepperoniThe Sac Daddy Root Beer Float 3.50 Of equal or lesser value. With this Cheese steak, fried egg & chopped bacon Pepper Jack cheese, steak, fried onions, Get 2 Cheese steak, egg, pork roll, scrapple & chopped bacon Doppler Dan’s Heatwave DESSERTS coupon. -
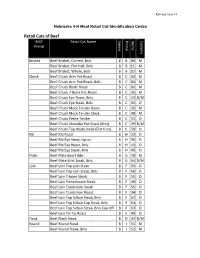
Retail Cuts of Beef BEEF Retail Cut Name Specie Primal Name Cookery Primal
Revised June 14 Nebraska 4-H Meat Retail Cut Identification Codes Retail Cuts of Beef BEEF Retail Cut Name Specie Primal Name Cookery Primal Brisket Beef Brisket, Corned, Bnls B B 89 M Beef Brisket, Flat Half, Bnls B B 15 M Beef Brisket, Whole, Bnls B B 10 M Chuck Beef Chuck Arm Pot-Roast B C 03 M Beef Chuck Arm Pot-Roast, Bnls B C 04 M Beef Chuck Blade Roast B C 06 M Beef Chuck 7-Bone Pot-Roast B C 26 M Beef Chuck Eye Roast, Bnls B C 13 D/M Beef Chuck Eye Steak, Bnls B C 45 D Beef Chuck Mock Tender Roast B C 20 M Beef Chuck Mock Tender Steak B C 48 M Beef Chuck Petite Tender B C 21 D Beef Chuck Shoulder Pot Roast (Bnls) B C 29 D/M Beef Chuck Top Blade Steak (Flat Iron) B C 58 D Rib Beef Rib Roast B H 22 D Beef Rib Eye Steak, Lip-on B H 50 D Beef Rib Eye Roast, Bnls B H 13 D Beef Rib Eye Steak, Bnls B H 45 D Plate Beef Plate Short Ribs B G 28 M Beef Plate Skirt Steak, Bnls B G 54 D/M Loin Beef Loin Top Loin Steak B F 59 D Beef Loin Top Loin Steak, Bnls B F 60 D Beef Loin T-bone Steak B F 55 D Beef Loin Porterhouse Steak B F 49 D Beef Loin Tenderloin Steak B F 56 D Beef Loin Tenderloin Roast B F 34 D Beef Loin Top Sirloin Steak, Bnls B F 62 D Beef Loin Top Sirloin Cap Steak, Bnls B F 64 D Beef Loin Top Sirloin Steak, Bnls Cap Off B F 63 D Beef Loin Tri-Tip Roast B F 40 D Flank Beef Flank Steak B D 47 D/M Round Beef Round Steak B I 51 M Beef Round Steak, Bnls B I 52 M BEEF Retail Cut Name Specie Primal Name Cookery Primal Beef Bottom Round Rump Roast B I 09 D/M Beef Round Top Round Steak B I 61 D Beef Round Top Round Roast B I 39 D Beef -

Oven Baked Beef Brisket with BBQ Sauce Video Above
Alternative recipes | RecipeTin Eats 22/2/19, 1157 am Oven Baked Beef Brisket with BBQ Sauce Video above. This is the oven baked version of the Slow Cooker Beef Brisket with BBQ Sauce. I've written it out as a separate recipe to make the recipe neater and easier to follow (rather than cramming it in the notes, as was previously done). Prep Time Cook Time Total Time 15 mins 10 hrs 10 hrs 15 mins Course: MainsCuisine: American, Southern Keyword: beef brisket, Brisket, Oven baked beef brisket Servings: 8 - 10 people Calories: 476kcal Author: Nagi Ingredients 1.5 – 2 kg / 3 – 4 lb beef brisket (Note 1) 1 tbsp olive oil (or a neutral oil like vegetable, canola) Rub: 1 tbsp brown sugar 2 tsp paprika powder 1 tsp onion powder 1 tsp garlic powder 1/2 tsp cumin powder 3/4 tsp mustard powder 1 tsp salt 1/2 tsp black pepper BBQ Sauce: 2 garlic cloves , minced 1/2 cup / 125 ml apple cider vinegar 1 1/2 cups / 375 ml ketchup 1/2 cup / 110g brown sugar , packed 2 tsp EACH black pepper , onion powder, mustard powder 1 tsp cayenne pepper (adjust to taste re: spiciness) 1 tbsp Worcestershire sauce Instructions 1. Place Rub in a bowl and mix to combine. Rub all over brisket. If time permits, leave for 30 minutes – 24 hours in the fridge, but I rarely do this. 2. Put the Sauce ingredients in a roasting pan, add 2 cups water. Mix, add beef, cover with lid or double layer of foil. -

Buldaegi Bbq House
BULDAEGI BBQ HOUSE TABLE GRILL Beverages Bottled Water — $2 Sparkling Water — $2.50 Hot Tea (Green or Earl Gray)— $1.75 Canned Soda — $1.75 Sweet or Unsweet Tea (2 refills) — $2.25 DOMESTIC BEER — $4 Yuengling Blue Moon IMPORTED BEER — $5 Tsingtao (China) Heineken (Holland) Asahi (Japan) Kirin Ichiban (Japan) Sapporo (Japan) OB (Korea) Fried Dumplings (튀김만두) — $8 Deep fried dumplings with chicken and vegetables. 8 pieces. Haemul Pancake (해물파전) — $16 Crispy pancake withAppetizers assorted seafood, carrot, green and white onion. House Japchae (불돼지잡채) — $14 Glass noodles, carrot, white and green onion. Choose a style: Pork, Beef, or Veggie. Duk Bok Ki* (떡볶기) — $14 Rice cake, fish cake, hard-boiled egg, hot pepper paste sauce.Spicy. Dak Gangjeong (닭강정) — $14 Crispy boneless fried chicken glazed with sweet, housemade sauce. Tang Su Yuk (탕수육) — $18 Deep fried meat or tofu in housemade sweet & sour sauce. Choose a style: Beef, Pork, or Tofu. Spring Rolls - $8 Shredded cabbage, carrots, tofu, onions. 6 pieces. *Consuming raw or undercooked meats, poultry, seafood, shellfish, or egg may increase your risk of food-borne illnesses. Choose a minimum of 2 BBQ orders or 1 Combo. Includes lettuce wraps, banchan, corn cheese, steamed egg*. Extra small sidesTable — $6 each. (SeeGrill back page for options) Pork Combo A — $50.50 Choose any 5 meats from Pork BBQ. Serves 2. Pork Combo B — $70.50 Choose any 7 meats from Pork BBQ. Serves 3-4. Beef Combo A — $65.50 Choose 2 Beef BBQ and 2 Premium Beef BBQ. Serves 2. Beef Combo B — $89.50 Choose 2 Beef BBQ and 3 Premium Beef BBQ. -

Jerky Page 1 of 4
Jerky Page 1 of 4 26 Lyerly St. Houston, TX 77022 713-691-2935 800-356-5189 Fax: 713-691-3250 Jerky is an all-time favorite snack and can be made from beef, wild game, fish, chicken or turkey. E.Coli Concerns: The only safe way to prepare jerky made of ground meat is to raise the internal temperature of the meat to 160°F. This is best accomplished by preheating the meat until the internal temperature is at least 160°F. for at least 5 minutes. Then you may process the jerky in the preferred manner. MEAT CHOICE Jerky is made from lean cuts of meat such as Venison, Beef Skirts, Eye of Round, Sirloin Tip, Brisket, Inside Round, Flank Steaks, Chicken or Turkey Breasts. Flank and Round steaks from beef or wild game seem to be the meat of choice for most jerky makers. You can also make jerky from ground meats using a jerky gun or jerky press. (Be sure to remove any fat or membrane from the meat.) CURE OR UNCURED METHODS* Jerky Meat With Cure - Can be cold smoked or dried at low temperatures (under 160°F.) without the risk of spoiling. Making jerky this way allows you to cold smoke jerky in just about any type smokehouse you can fashion. Cure also adds it own unique flavor to the final taste. Jerky Meat Without Cure - Must be cooked or processed at temperatures above 160°F. This method of making jerky has to be done in ovens or smokers that allow the temperature to be regulated and maintained above 160°F. -

JUNETEENTH RECIPES from the SWEET HOME CAFÉ
JUNETEENTH RECIPES from the SWEET HOME CAFÉ BABARBECUED R B E C U E D BEEF BR ISK E T S A ND W I C H BEEF BRISKET SANDWICH to 6 8 A C TIV E TI M E IN MUCH OF THE SOUTH, 1 (5-pound) beef brisket, trimmed 4 pounds natural wood charcoal 25 MINS barbecue is about pork. In Texas, of excess fat (leave ¼-inch fat cap on 6 to 8 artisanal sandwich buns, the meat) for serving however, beef brisket is the chosen 1 DAY meat on the barbecue trail. The 1 cup Barbecue Dry Rub (page 196) Kosher salt 1 tablespoon coarse sea salt OV ER N I G H T tender, smoky beef pairs well Freshly ground black pepper SO AKING 3 tablespoons cracked black OF T H E with charred peach chutney in Charred Peach & Jalapeño peppercorns the Café’s version of a barbecued Chutney (page 136), for serving beef brisket sandwich. 10 pounds hickory wood chunks THE NIGHT BEFORE Generously season the brisket on all sides with the Barbecue Dry Rub, sea salt, and peppercorns. Rub them thoroughly into the meat, fat, and pockets to ensure even distribution. Place the brisket on a wire rack set on top of a baking sheet. Place uncovered in the refrigerator for 12 hours to marinate. Cover the hickory chunks with water and leave to soak overnight. THE NEXT DAY Remove the brisket from the refrigerator and let it to come to room temperature. Drain the hickory chunks. To set up your smoker, first ignite the charcoal. -

Beef Cut Sheet
Beef Cut Sheet Visit www.edgewoodlocker.com to submit your order online or Call (563) 928 -6814 to talk with our experienced staff. 609 West Union Street Rump or Rolled Rump Roast P.O. Box 245 Ribeye Round Steak Steak Edgewood, Iowa 52042 Tenderized Round Steak or (563) 928-6814 T-Bones Minute Steak Bone-In www.edgewoodlocker.com or Dried Beef Ribeye New York Strips & Filets Beef Jerky Prime Rib Sliced BBQ Beef Arm Roast Sliced Roast Beef & Gravy Chuck Roast Chuck-eye Steak Shredded BBQ Beef Shredded Roast Beef & Gravy Soup Bones Brisket Beef Bacon Top Sirloin Steak Sirloin Tip Roast Trimmings Flank Steak Or or Trimmings Top Sirloin Roast Short Ribs Trimmings Frequently Asked Questions Q: How thick should I cut my steaks? A: Average is ¾ inch thick, but can do thicker or thinner based on personal preference Q: How many steaks should I put in a package? A: Average is 2, but depends on personal preference Q: What’s the difference between a tenderized round steak and a minute steak? A: The tenderized round steak goes through the tenderizer 2-3 times averaging roughly 1 pound and the minute steak goes through 4-5 times averaging roughly 1/3 pound Q: How many pounds should my roasts be? A: Average is 3 pounds, but varies based on personal preference Q: What size packages of hamburger should I get? A: You can select 1, 1½ or 2 pound packages Q: I don’t want ribs, brisket, soup bones, or stew meat. What happens to that meat? A: These will be trimmed and become ground beef or sausage items Q: I don’t know a farmer but still want beef, what do I do? A: We have beef delivered from a local farmer for your purchase. -

Beef Cuts for Portioning
BEEF CUTS FOR PORTIONING CHUCK RIB LOIN SIRLOIN ROUND TOP SIRLOIN STEAK CHUCK ROLL PRIME RIB SHORT LOIN 1184 Beef Loin, Top Sirloin Butt Steak, Boneless STEAMSHIP ROUND 116A Beef Chuck, Chuck Roll 109E Beef Rib, Ribeye Roll, Lip-On, Bone In (Export Style) 174 Beef Loin, Short Loin, Short-Cut 166B Beef Round, Rump and Shank Partially Off, Handle On ORDER SPECIFICATIONS ORDER SPECIFICATIONS ORDER SPECIFICATIONS ORDER SPECIFICATIONS • Quality grade ORDER SPECIFICATIONS • Quality grade • Quality grade • Quality grade • Thickness or portion weight • Quality grade • Different arm length portion • Fat cover • Length of tail • Thickness of surface fat • Removal of shank meat exclusions - ventral cut • Weight range • Thickness of surface fat • Specify 1184A to purchase without • Thickness of surface fat • Removal of subscapularis • Thickness of surface fat • Weight range the gluteus accessorius and Portioned Top • Portion weight: 30 to 50 pounds Sirloin Steak Cooking method: Moist heat • Length of tail (lip) Cooking method: Dry heat gluteus profundus Cooking method: • Tied or netted • Specify 1184B to purchase center-cut Dry heat – roast (Cap off) – gluteus medius muscle only Cooking method: Dry heat Cooking method: Dry heat CHUCK EYE STEAK PORTERHOUSE STEAK 1116D PSO:1 Beef Chuck, Chuck Eye Roll Steak, Boneless 1173 Beef Loin, Porterhouse Steak ORDER SPECIFICATIONS RIB STEAK ORDER SPECIFICATIONS 1103 Beef Rib, Rib Steak, Bone In TOP SIRLOIN FILET • Prepared from item 116D • Quality grade 1184F Beef Loin, Top Sirloin Butt Steak, Center-Cut, Boneless, -

Unlimited Korean
UNLIMITED KOREAN BBQ Everyone at the table must participate and order the same option Must be 2 Adults+ (No singles for Unlimited BBQ) 1 Hour 30 min Maximum Grill Time Last Call is 20 minutes before time limit Served with Rice, Side Dishes, Lettuce & Dipping Sauces Must order four different types of meats on the first round Option A Adults $23.99 Ages 4-8 $11.99 Kids 3 & Under FREE 1. Samgyupsal 삼겹살 Pork belly 2.Chadolbaegi 차돌배기 Beef brisket 3. Beef Bulgogi 불고기 Beef marinated in sweet soy sauce 4. Chicken Bulgogi 닭불고기 Chicken marinated in sweet soy sauce 5. Spicy Chicken (spicy) 매운 닭불고기 Spicy chicken thigh 6. Dwaeji Bulgogi (spicy) 돼지 불고기 Thinly sliced pork w/spicy sauce Option B Adults $28.99 Ages 4-8 $14.49 Kids 3 & Under FREE 1. Samgyupsal 삼겹살 Pork belly 2.Chadolbaegi 차돌배기 Beef brisket 3. Beef Bulgogi 불고기 Beef marinated in sweet soy sauce Extras 4. Chicken Bulgogi 닭불고기 Coke, Diet Coke, Sprite, Mr. Pibb $2.99 Chicken marinated in sweet soy sauce Extra Rice bowl $1.99 5. Spicy Chicken (spicy) 매운 닭불고기 Rice Cake Sliced $1.99 Spicy chicken thigh Rice Cake Wraps $1.99 6. Dwaeji Bulgogi (spicy) 돼지 불고기 Spicy Pork Sliced Potato FREE 치마살 7. Skirt Steak Kabocha (sweet squash) FREE Fresh cut skirt steak Mushroom FREE 8. Galbi 갈비 Sweet Potato FREE Our famous Sweet soy marinated Short Ribs Steamed Egg or Doenjang Jjigae FREE/per table 9. Galbi Jumulluck 갈비 주물럭 Beef short ribs seasoned with salt, pepper & sesame oil Leftover Charge $10 per person No togo boxes for leftovers given Consuming raw or undercooked meats, poultry, 18% Gratuity for Parties of 6+ seafood, shellfish, or eggs may increase your risk of foodborne illness Max check split limited to 4 cards Option C Adults $31.88 Ages 4-8 $15.99 Kids 3 & Under FREE 1. -

Chicken Chicken & Veggies Brisket Meatballs Filet Mignon Oxtail
small plates phở pork egg rolls 5 pork, carrots, mushrooms, glass noodles, served with fish sauce (2) vegetable egg rolls 5.5 taro, carrots, mushrooms, edamame, glass noodles, served with sweet chili sauce (3) shrimp spring rolls 5 shrimp, lettuce, sprouts, vermicelli, wrapped in rice paper, served with peanut sauce (2) grilled pork spring rolls 5 charbroiled pork, lettuce, sprouts, vermicelli, wrapped in rice paper, served with peanut sauce (2) tofu spring rolls 4.5 tofu, lettuce, sprouts, vermicelli, wrapped in rice paper, served with peanut sauce (2) add a fountain drink + egg roll for only $3 Slow cooked bone broth, rice noodles, red & green onion, cilantro. additional toppings available at garnish station (dine-in only). sub zuchinni noodles $1.50 chicken 9.50 chicken & veggies 9.50 brisket 10 meatballs 10 filet mignon 11.95 “bánh mi” sliders 6 oxtail 12.95 charbroiled pork or crisp tofu, house aioli, pickled carrots, cucumber, jalapeno, vegetarian 9.50 cilantro, in a steamed “bao” bun* (2) seafood 10.95 shrimp 10.95 crispy salt and pepper tofu 6.5 fried tofu, roasted garlic, pickled carrot, eye round steak* 10 cilantro, house spices, served with sweet chili sauce eye round steak / tendon* 10 eye round steak / brisket* 10 ask about our gluten-free eye round steak / meatballs* 10 and vegan options no meat (choice of broth) 7.50 (v) vegetarian * (disclaimer) consuming raw or undercooked meats, poultry, seafood, shellfish, or eggs may increase your risk of foodborne illness PhởNatic specials bánh mi sandwiches phở-nomenal combo 11.95 -

4Generations
www.McDonaldsMeats.com Clear Lake, Minnesota Full-Service Meat Counter Meat Full-Service GENERATIONS Beef Steaks Ribeye Steak Turkey since 1914 Bone-In Ribeye Steak Ground Beef 2012 GRAND CHAMPION 4 Quantity Discounts Available on Ground Beef New York Strip Steak SMOKED TURKEY T-Bone Steak 85% Lean Ground Beef Special Cuts Boneless Turkey Breast Fillets 93% Extra Lean Ground Beef Lemon Peppered Boneless Turkey Breast As a USDA inspected facility, we are committed to offering quality products and Porterhouse Steak Tenderloin Steak 85% Locally Raised Lean Ground Beef Fillets exceptional customer service. Through four generations, we’ve kept our dedication Top Sirloin Steak Ground Beef Patties Ground Turkey to quality and service. Today, our meat market features a full-service meat counter, Ball Tip Steak Mushroom & Onion Ground Beef Patties Ground Turkey Patties special cuts, as well as homemade sausages and our world-famous jerky. Mac’s Seasoned Sirloin Steak Mushroom & Swiss Ground Beef Patties Pork Stuffed Turkey Rolls Montreal Seasoned Steak Wild Rice Ground Beef Patties Pork Chops Frozen Turkeys Visit our online store at Flat Iron Steak Bacon Ground Beef Patties Thick Cut Pork Chops McDonaldsMeats.com Jalapeno Cheddar Ground Beef Patties BBQ Seasoned Pork Chops GIFT CARDS available Round Steak Chicken Visa, Master Card, Discover, American Flank Steak Pizza Burgers Teriyaki Pork Chops Cut-Up Chicken Fryers Express and EBT cards accepted! Chuck Eye Steak BBQ Bacon Cheeseburger Patties Boneless Pork Chops Home Grown Chickens We process all types of Greek Seasoned Skirt Steak Black & Bleu Burgers Pork Roast Boneless Chicken Breast wild game all year long.