Status of Knowledge and Skills of Cold Chain Handlers in District Meerut, Uttar Pradesh, India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CP Vol VIII EIA
GOVERNMENT OF UTTAR PRADESH Public Works Department Uttar Pradesh Core Road Network Development Program Part – A: Project Preparation DETAILED PROJECT REPORT Volume - VIII: Environmental Impact Assessment Report and Environmental Management Plan Gola – Shahjahanpur Road (SH-93) July 2015 India Consulting engineers pvt. ltd. Uttar Pradesh Core Road Network Development Program DETAILED PROJECT REPORT Volume-VIII: EIA and EMP Report Gola – Shahjahanpur Road (SH-93) Volume-VIII : Environmental Impact Assessment Report (EIA) and Document Name Environmental Management Plan (EMP) (Detailed Project Report) Document Number EIRH1UP020/DPR/SH-93/GS/004/VIII Uttar Pradesh Core Road Network Development Program Project Name Part – A: Project Preparation including Detailed Engineering Design and Contract Documentation Project Number EIRH1UP020 Document Authentication Name Designation Prepared by Dr. S.S. Deepak Environmental Specialist Reviewed by Sudhendra Kumar Karanam Sr. General Manager (Roads & Highways) Rajeev Kumar Gupta Deputy Team Leader Avadesh Singh Technical Head Approved by Rick Camise Team Leader History of Revisions Version Date Description of Change(s) Rev. 0 19/12/2014 First Submission Rev. 1 29/12/2014 Second Submission after incorporating World Bank’s Comments and Suggestions Rev. 2 13/01/2015 Incorporating World Bank’s Comments and Suggestions Rev. 3 16/07/2015 Revision after discussion with Independent Consultant Page i| Rev: R3 , Uttar Pradesh Core Road Network Development Program DETAILED PROJECT REPORT Volume-VIII: EIA and EMP -
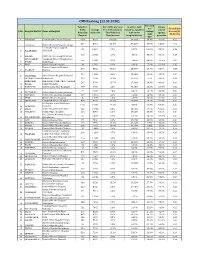
CMS Ranking (12.03.2020) Percentil Total Pre- Percentile of ( % of % of Pre-Auth Claims E of Consolidate Auths Average Pre‐Auth Raised Vs Raised Vs
CMS Ranking (12.03.2020) Percentil Total Pre- Percentile of ( % of % of Pre-Auth Claims e of Consolidate Auths Average Pre-Auth raised vs raised vs. total Pre- raised S.No. Hospital District Name of Hospital average d score (30- Raised by claim size Total District) Auth in the against claim 30-30-10) Hospital Beneficiaries hospital district preauths size 1 VARANASI Homi Bhabha Cancer Hospital 4456 8325 100.0% 16.61% 36.3% 92.1% 0.55 560 8691 31.3% 37.31% 37.9% 98.8% 0.42 2 LALITPUR District Female Hospital Lalitpur Tej Bhadur Sapru Hospital 81 22910 1.3% 0.58% 100.0% 88.9% 0.39 3 ALLAHABAD Allahabad 123 20157 2.1% 4.54% 88.0% 98.4% 0.38 4 HARDOI District Female Hospital Hardoi SANT KABEER Combined District Hospital Sant 66 19332 3.1% 2.65% 84.4% 100.0% 0.37 5 NAGAR Kabir Nagar 6 MAU Distric Female Hospital 39 17641 1.6% 6.31% 77.0% 100.0% 0.35 District Combined Hospital 374 6352 41.5% 15.56% 27.7% 98.9% 0.35 7 BAGHPAT Baghpat 204 14262 3.9% 15.94% 62.3% 98.5% 0.34 8 RAE BARELI Rbms District Hospital Raebareli 9 SIDDHARTH NAGADchsdr.001 305 2089 10.3% 62.37% 9.1% 98.0% 0.34 AMBEDKAR Mmahatma Jyotiba Phule Combind 517 8784 15.7% 27.33% 38.3% 98.6% 0.34 10 NAGAR District Hospital 11 HAMIRPUR Distt Hospital Male Hamirpur 107 5786 6.2% 43.15% 25.3% 100.0% 0.32 72 16992 2.3% 3.37% 74.2% 83.3% 0.32 12 SULTANPUR District Male Hospital Sultanpur 13 FAIZABAD District Haopita Male Faizabad 117 15746 3.2% 2.65% 68.7% 97.4% 0.32 14 ETAH District Women Hospital 5 17875 0.3% 0.77% 78.0% 80.0% 0.32 15 SHRAVASTI Combined District Hospital 484 3224 31.6% -

Ubrief Industrial Profile of Shahjahanpur District
Government of India Ministry of MSME BriefU Industrial Profile of Shahjahanpur District U.P MSME-Development Institute (Ministry of MSME, Govt. of India,) Phone: 0512-2295070-73 Fax: 0512-2240143 E-mail : [email protected] Web- msmedikanpur.gov.in 1 FOREWORD District Industrial Potentiality Survey Report of District Shahjahnpur is a key report which not only contains current industrial scenario of the district but also other useful information about the district. This report provides valuable inputs which may be useful for existing & prospective entrepreneurs of the District. It is the only source which provides the latest data on infrastructure, banking and industry of the district. It also provides information on potentials areas in manufacturing and service sector of the district. District Administration, District Industries and Enterprise Promotion Centre, Shahjahanpur, Statistical Department, Agriculture & Horticulture Offices, District Lead Bank, Live Stock Office and other departments of Shahjahanpur and Lucknow have provided valuable information via journal, website, telephonic conversation and other communication channels. I sincerely hope that District Industrial Potentiality Survey Report of District Shahjahanpur will facilitate easier dissemination of information about the district to policy makers and also to the professionals working in the MSME sector. I appreciate the efforts made by Shri Vijay Kumar Bhatt, Asstt. Director (Stat.) in preparing the District Industrial Potentiality Survey Report of Shahjahnpur District. June, 2016 ( U. C. Shukla ) Kanpur Director 2 Contents S. No. Topic Page No. 1. General Characteristics of the District 03 1.1 Location & Geographical Area 03 1.2 Topography 03 1.3 Availability of Minerals. 04 1.4 Forest 04 1.5 Administrative set up 04 2. -

Project Site Location Justification Note
Project Site Location Justification Note Proposed Protected forest land to be diverted for Doubling of Railway Track from (Km No.- 0.00 to 80.300) Roza to Sitapur Railway {KM No.- 0.00 – 11.00 in Shahjahanpur district} in Northern Railway Division Moradabad, this is to further certify that the forest area involved in the proposal is unavoidable and barest minimum total forest area 8.80 Ha is proposed for diversion. Mahesh Chand Executive Engineer/Con Northern Railway,Moradabad Project Site Location Justification Note Proposed Protected forest land to be diverted for Doubling of Railway Track from (Km No.- 0.00 to 80.30) Roza to Sitapur Railway {KM No.- 11.00 to 28.00 & 34.00 to 46.00 in Lakhimpur Kheri district} in Northern Railway Division Moradabad, this is to further certify that the forest area involved in the proposal is unavoidable and barest minimum total forest area 24.06 Ha is proposed for diversion. Mahesh Chand Executive Engineer/Con Northern Railway,Moradabad Project Site Location Justification Note Proposed Protected forest land to be diverted for Doubling of Railway Track from (Km No.- 0.00 to 80.30) Roza to Sitapur Railway {KM No.- 28.00 to 34.00 in Hardoi district} in Northern Railway Division Moradabad, this is to further certify that the forest area involved in the proposal is unavoidable and barest minimum total forest area 6.1133 Ha is proposed for diversion. Mahesh Chand Executive Engineer/Con Northern Railway,Moradabad Project Site Location Justification Note Proposed Protected forest land to be diverted for Doubling of Railway Track from (Km No.- 0.00 to 80.30) Roza to Sitapur Railway {KM No.- 46.00 to 80.30 in Sitapur district} in Northern Railway Division Moradabad, this is to further certify that the forest area involved in the proposal is unavoidable and barest minimum total forest area 30.6259 Ha is proposed for diversion. -

Uttar Pradesh
DLHS -2 DLHS-2 Uttar Pradesh Reproductive and Child Health District Level Household Survey 2002-04 International Institute for Ministry of Health & Family TNS India Private Limited Population Sciences Welfare, Government of India New Delhi – 110 016 (Deemed University) New Delhi – 110 011 Mumbai – 400 088 Reproductive and Child Health District Level Household Survey (DLHS - 2) Uttar Pradesh 2002-04 International Institute for Ministry of Health & Family Population Sciences, Welfare, Government of India, TNS India Pvt. Ltd., (Deemed University) New Delhi – 110 011 New Delhi – 110 016 Mumbai – 400 088 Contributors TNS India Private Limited, New Delhi Tilak Mukherji U.V.Somayajulu S. Anil Chandran B. S. Singh International Institute for Population Sciences, Mumbai F. Ram B. Paswan L. Ladu Singh Rajiv Ranjan Akash Wankhede CONTENTS Page Tables ................................................................................................................................ iv Figures ................................................................................................................................ vii Maps ................................................................................................................................... vii Preface and Acknowledgement ......................................................................................... ix Key Indicators ................................................................................................................... xi Salient Findings ................................................................................................................ -

For Award of Ph.D /D.Litt./D.Sc. DEGREE Ph.D. Fnukad 26&5&2012 Ls 18&10&2012 Rd Leiuu Gqbz Eksf[Kdh Ijh{Kkvksa
For Award of Ph.D /D.Litt./D.Sc. DEGREE fnukad 26&5&2012 ls 18&10&2012 rd lEiUu gqbZ ekSf[kdh ijh{kkvksa dk fooj.k Ph.D. Effect of Advermectins on Haematology and Enzyme Profiles of Fresh Water Fish 1 Zoology Namita Verma Dr. K.K.Saxena R-0130295 H.No.108-B,KrishnaNagar Bareilly College 26.5.2012 Izatnagar, Bareilly Bareilly. 2 Geography Jasneet Kaur Dr. R.P.Singh Patterns and Dynamics of Agricultural Land Use in Shahjahanpur District R-08112429 69-Green Park Vistar Principal, 26.5.2012 Bareilly Bareilly College.Bareilly 3 Economics Sandhya Jaiswal Dr. N.L.Sharma mRrj izns'k dh ifjogu O;oLFkk dk fo'ys"k.kkRed v/;;u R-06113623 245-Bagh Ahmad Ali Bareilly College 26.5.2012 Talab, Bareilly Bareilly 4 Physics Nupur Saxena Dr, Avinash Agarwal Studies of Embedded Silicon Nanocrystals Formation by Energetic Ion Beam R-9825946 405/F, Kunwarpur(Sivpuri) Bareilly College 26.5.2012 Bareilly Bareilly 5 English Poonam Rani Dr. Santosh Agrawal The Function of Imagery in Graham Greene's Major Novels : A Critical Study R-9810296 Chauraha Gali Near Devi G.D.H.G.College 26.5.2012 Ji Ka Mandir,Moradabad Moradabad 6 Computer Sc. Mano Yadav Dr. K.V.Arya Study of Routing Issues in Mobile AD HOC Networks R-0126844 426-Beharipur Civil Lines ABV- IIITM 28.5.2012 Bareilly Gwalior 7 Education Rajeev Kumar Singh Dr. Shabhat Jeet Singh izkFkfed fo|ky;ksa esa dk;Zjr f'k{kkfe=ksa dh f'k{k.k ds izfr vfHko``fRr ,oa dk;Z larqf"V dks izHkkfor djus okys dkjdksa dk v/;;u R-0565366 D-27-9 South City S.S.College 28.5.2012 Shahjahanpur Shahjahanpur 8 Sanskrit Piyush Sharma Dr. -

Project Summary
Project Summary Proposed Protected forest land to be diverted for Doubling of Railway Track from (Km No.- 0.00 to 80.30) Roza to Sitapur Railway {KM No.- 0.00 – 11.00 in Shahjahanpur district}, {KM No.- 11.00 to 28.00 & 34.00 to 46.00 in Lakhimpur Kheri district}, {KM No.- 28.00 to 34.00 in Hardoi district} and {KM No.- 46.00 to 80.30 in Sitapur district} in Northern Railway Division Moradabad. District wise details of forest land and non forest land are as follows: Sr. No. DISTRICT PROTECTED NON FOREST FOREST LAND LAND Area (in Ha) Area (in Ha) 1 Shahjahanpur 8.80 0.0175 2 Lakhimpur Kheri 24.06 0.3235 3 Hardoi 6.1133 0.0851 4 Sitapur 30.6259 0.6435 Total Area 69.5992 1.0696 Mahesh Chand Executive Engineer/Con Northern Railway,Moradabad Area Calculation Sheet Proposed Protected Forest Land to be Diverted For Proposed new 2nd line along with existing Railway line under Roza - Sitapur Railway Track (Km. no. 0.00 - 80.30) Doubling Project of Northern Railway, from Km. 0.00 to 11.00, in Moradabad Division, District: Shahjahanpur (Uttar Pradesh) Protected Forest Land Area Calculation From To Length Width Area Area Sr. No. (Km) (Km) (m) (m) (SqM) (Ha) 1 1.900 7.360 5460.00 8.00 43680.00 4.3680 2 8.670 11.000 2330.00 8.00 18640.00 1.8640 7790.00 62320.00 6.2320 Proposed Protected Forest Land Area 6.2320Ha Protected Forest Land along Railway Yards Forest Land Area Calculation From To Length Width Area Area Sr. -

Village Survey Monograph No-1, Woollen Carpet and Blanket Industry, Part VII-A, Vol-XV, Uttar Pradesh
PR G. 141. A. eN) (Ordy) 775 CENSUS OF INDIA, 1961 VOLUME XV UTTAR PRADESH PART VII A HANDICRAFTS SURVEY MONOGRAPH NO. 1 General Editor P.P.BHATNAGAR of the Indian Administrative Service Superintentlent of Census Opn-alions, Uttar Pradesh Y' , W-OOLLEN CARPET AND BLANKET INDUSTRY IN UTTAR PRADESH with speoial study of WOOLLEN CARPET INDUSTRY AT SHAHJAHANPUR AND BLANKET INDUSTRY AT MUZAFFARNAGAR BY R. C. SHARMA of the Uttar Pradesh Civil Service Deputy Superintendent of Census Operations PRINTED IN INDIA BY THE SUPDT., PRINTING & STY., u.p. AT JOB PRINTERS, ALLAHABAD & PUBLISHED BY THE M\NAGER OF PUBLICATIONS, DELHI-8 1964 Price : (Inland) Rs. 5'60 P (Foreign) 13 sh. 1 d. or 2 $ 2 cents. CENSUS OF INDIA. 1961 Central Government Publications Census Report. Volume XV-Uttar Pradesh is published in the following Parts:- I-A (i-ii) General R~port I-B Report 011 Vital Statistics I-C (i-v) Subsidiary Tables II-A General Population Tables U"'-:'B (i-vii) General Ecbnomic Tables II-C (i-vi) Cultural and Migration Tables I II-A Household Economic Tables III-B Household Economic Tables (concluded) IV-~ Report on Housing and Establishments alld Housing and Establishment Tables (E-series Tables-except E-III) IV-B Housing and Estab)ishment Tables (E-III) V-A Special Tables for Scheduled Castes V-B Reprints from old Census Reports and Ethnographic Notes VI Village Survey Monographs (Monographs on Selected Villages) VII~A Handicraft Survey Reports VII--B Fairs and Festivals in Uttar Pradesh VIII-A Administration Report on Enumeration (for official use only) VIII-B Administration Report on Tabulation (for official we only) IX Census Atlas of Uttar Pradesh X Special Report on Kanpur State Government Publications 54 Volumes of District Census Handbooks CONTENTS Page Foreword Preface iv SECTION I WOOLLEN CARPET AND BLANKET INDUSTRY IN UTT AR PRADESH SECTION II WOOLLEN CA,RPET INDUSTRY AT SHAHJAHANPUR ChaPt~r I. -

An Economic Analysis of Production of Sugarcane In
International Journal of Agriculture and Allied Sciences, 4(2): 5-8, 2019 AN ECONOMIC ANALYSIS OF PRODUCTION OF SUGARCANE IN SHAHJAHANPUR DISTRICT OF UTTAR PRADESH Mukul Sankhwar1 and Ramachandra2 Department of Agricultural Economics, Sam Higginbottom University of Agriculture, Technology and Sciences, Prayagraj-211007, Uttar Pradesh, India ABSTRACT: The present study was conducted in Bhawal Khera block in Shahjahanpur district of U.P state. 100 farmers were selected randomly from five villages. Out of 100 farmers 45 small, 33 medium and 22 large farmers were selected from study area .The primary data were collected for the year 2017-2018. To calculate the cost of cultivation, marketable surplus and disposable pattern of sugarcane simple mean and average method was used. The major findings of this study revealed that the average holding size of the sampled household was 1.78 hectares and average illiteracy percentage was 80.88 percent. Overall on an average cropping intensity was found 250 percent. The major crops grown by the farmers were wheat and sugarcane in kharif and vegetables in Rabi season. On an average the total irrigated area of sampled household was found 56.22 percent and the maximum irrigated area comes under tube well 0.52 hectares (59.29 percent).The production performance of sugarcane in overall period (2001-2013) was observed positive and significant growth in state of U.P, Lakhimpur (Kheri) district have also shows the positive growth rate of area and production. On an average cost of cultivation per hectare of sugarcane was found Rs. 67187.02. The value of output on an average was found to be Rs. -

Changes in Cropping Pattern in Shahjahanpur District- Uttar Pradesh
International Journal of Geography, Geology and Environment 2019; 1(1): 41-47 P-ISSN: 2706-7483 E-ISSN: 2706-7491 IJGGE 2019; 1(1): 41-47 Changes in cropping pattern in shahjahanpur district- Received: 19-05-2019 Accepted: 23-06-2019 Uttar Pradesh Nida Bee Research Scholar, Department Nida Bee and Dr. Fazlur Rahman of Geography, Aligarh Muslim University Aligarh, Uttar Pradesh, India Abstract Agriculture plays a vital role in the economic development of the country where about 70 percent of Dr. Fazlur Rahman the population is directly or indirectly depends on agricultural activities. The agriculture sector is still Assistant Professor, the major source of livelihood for the population of Shahjahanpur district. With the advancement of Department of Geography, new agricultural strategy, there has been a complementary relationship between the commercialization Aligarh Muslim University and cropping pattern of the state. The present study aims that understanding the dynamics of Aligarh, Uttar Pradesh, India agricultural development by utilizing the secondary sources of data which is collected from various government publications. The area under different crops has been analyzed and interpreted through tabulation and simple percentage method. The aim of this paper is to find out the changes in the cropping pattern in Shahjahanpur district. The initial analysis of the data reflects that the farmers are gradually diversifying their farms and intended to shift towards cash crops for the sake of higher returns. The area under commercial crops in the district has been increasing at a higher rate as compared to other food crops. Keywords: Shahjahanpur, cropping pattern, food crops, commercialization Introduction Cropping pattern means the share of area under different crops at a particular period of time. -

ISSN -2347-856X ISSN -2348-0653 International
Research Paper IJBARR Impact Factor: 3.072 E- ISSN -2347-856X Peer Reviewed, Listed & Indexed ISSN -2348-0653 A STUDY OF WOMEN EMPOWERMENT IN BAREILLY REGION OF U.P Dr. Deepak Misra S.S.(PG) College, Shahjahanpur Abstract In the study area women workers are not only illiterate but are ignorant also. Whatever skills have been acquired by some of them is outside the formal school system. Without any job experience, they enter as new entrants. There small establishments employ casual workers with no job security. The size of employment in these establishments is very small. There is a single worker in some of the self-employed establishments. Local inputs are mostly used by these establishments because of small sector operations. There is no structural organization in family ownership enterprises in the study area. It has been observed that in many cases, the head of the family or a member of the family does the work himself with the help of other members of the family. In these enterprises there is neither an employee nor an employer. Keywords : Illiterate, Employment Family. INTRODUCTION India is a developing country and near about 67% of its population lived in rural area. Since 1947 to 2013 the population of India increased many times but according the population literacy rate, growth rate and banking services in rural area has not increased. Uttar Pradesh literally translated as “Northern Province” in English is a state situated in northern India. Lucknow is the capital of Uttar Pradesh and Kanpur is economic and industrial capital of Uttar Pradesh.The state is surrounded by the country of Nepal and Uttarakhand to the north, Delhi and Haryana to the northwest, Rajasthan to the West, Madhya Pradesh to the south west, and Bihar to the East and Jharkhand to the southwest. -

High Court of Judicature at Allahabad (Tender Reference No
High Court of Judicature at Allahabad (Tender Reference No. : AHC/e-Courts/Desktop/01/2016) HIGH COURT OF JUDICATURE AT ALLAHABAD, ALLAHABAD (U.P.) - 211001 NOTICE INVITING ONLINE TENDERS FOR AWARD OF CONTRACT FOR SUPPLY, TESTING, INSTALLATION AND ONSITE COMPREHENSIVE WARRANTY SUPPORT OF DESKTOP COMPUTERS AT THE DISTRICT COURTS SUBORDINATE TO THE HIGH COURT OF JUDICATURE AT ALLAHABAD , UNDER E- COURTS PROJECT. 1 High Court of Judicature at Allahabad (Tender Reference No. : AHC/e-Courts/Desktop/01/2016) High Court of Judicature at Allahabad invites ONLINE Bids only, in two Bid system, from eligible and reputed Original Equipment Manufacturers (OEM's) of Desktop Computers and/or their authorized channel partners and/or dealers/ agents/ suppliers of Desktop Computers for award of contract for supply, testing, installation and onsite comprehensive warranty support of Desktop Computers at District Courts subordinate to the High Court of Judicature at Allahabad, under e-Courts Project. The tender document has been published on the e-Procurement/ e-Tendering System of the Government of Uttar Pradesh at https://etender.up.nic.in and the official website of the Allahabad High Court at http://www.allahabadhighcourt.in. The interested and eligible Bidders may submit soft copies of their Bids electronically through e-Tendering System of the Government of Uttar Pradesh at https://etender.up.nic.in , using a valid Digital Signature Certificates. The detailed information for submitting online Bids through e-Tendering System may be obtained from the e-Procurement/ e-Tendering System of the Government of Uttar Pradesh at https://etender.up.nic.in . Bids are required to be submitted with a tender fee (non-refundable) of Rs.