Redalyc.Hydrogeochemical Attribut Es and Ground Water Quality of Ngbo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

South – East Zone
South – East Zone Abia State Contact Number/Enquires ‐08036725051 S/N City / Town Street Address 1 Aba Abia State Polytechnic, Aba 2 Aba Aba Main Park (Asa Road) 3 Aba Ogbor Hill (Opobo Junction) 4 Aba Iheoji Market (Ohanku, Aba) 5 Aba Osisioma By Express 6 Aba Eziama Aba North (Pz) 7 Aba 222 Clifford Road (Agm Church) 8 Aba Aba Town Hall, L.G Hqr, Aba South 9 Aba A.G.C. 39 Osusu Rd, Aba North 10 Aba A.G.C. 22 Ikonne Street, Aba North 11 Aba A.G.C. 252 Faulks Road, Aba North 12 Aba A.G.C. 84 Ohanku Road, Aba South 13 Aba A.G.C. Ukaegbu Ogbor Hill, Aba North 14 Aba A.G.C. Ozuitem, Aba South 15 Aba A.G.C. 55 Ogbonna Rd, Aba North 16 Aba Sda, 1 School Rd, Aba South 17 Aba Our Lady Of Rose Cath. Ngwa Rd, Aba South 18 Aba Abia State University Teaching Hospital – Hospital Road, Aba 19 Aba Ama Ogbonna/Osusu, Aba 20 Aba Ahia Ohuru, Aba 21 Aba Abayi Ariaria, Aba 22 Aba Seven ‐ Up Ogbor Hill, Aba 23 Aba Asa Nnetu – Spair Parts Market, Aba 24 Aba Zonal Board/Afor Une, Aba 25 Aba Obohia ‐ Our Lady Of Fatima, Aba 26 Aba Mr Bigs – Factory Road, Aba 27 Aba Ph Rd ‐ Udenwanyi, Aba 28 Aba Tony‐ Mas Becoz Fast Food‐ Umuode By Express, Aba 29 Aba Okpu Umuobo – By Aba Owerri Road, Aba 30 Aba Obikabia Junction – Ogbor Hill, Aba 31 Aba Ihemelandu – Evina, Aba 32 Aba East Street By Azikiwe – New Era Hospital, Aba 33 Aba Owerri – Aba Primary School, Aba 34 Aba Nigeria Breweries – Industrial Road, Aba 35 Aba Orie Ohabiam Market, Aba 36 Aba Jubilee By Asa Road, Aba 37 Aba St. -

Prevalence of Paragonimus Infection
American Journal of Infectious Diseases 9 (1): 17-23, 2013 ISSN: 1553-6203 ©2013 Science Publication doi:10.3844/ajidsp.2013.17.23 Published Online 9 (1) 2013 (http://www.thescipub.com/ajid.toc) Prevalence of Paragonimus Infection 1Nworie Okoro, 2Reginald Azu Onyeagba, 3Chukwudi Anyim, 4Ogbuinya Elom Eda, 3Chukwudum Somadina Okoli, 5Ikechukwu Orji, 3Eucharia Chinyere Okonkwo, 3Uchechukwu Onyeukwu Ekuma and 3Maduka Victor Agah 1Department of Biological Science, Faculty of Science and Technology, Federal University Ndufu Alike-Ikwo, Nigeria 2Department of Microbiology, Faculty of Biological and Physical Sciences, Abia State University, Uturu-Okigwe, Nigeria 3Department of Applied Microbiology, Faculty of Biological Science, Ebonyi State University, Abakaliki, Nigeria 4Hospital Management Board, Ministry of Health, Abakaliki, Nigeria 5Federal Teaching Hospital Abakaliki II (FETHA II), Abakaliki, Ebonyi, Nigeria Received 2013-01-25, Revised 2013-02-19; Accepted 2013-05-20 ABSTRACT Paragonimiasis (human infections with the lung fluke Paragonimus westermani ) is an important public health problem in parts of Africa. This study was aimed at assessing the prevalence of Paragonimus infection in Ebonyi State. Deep sputum samples from 3600 individuals and stool samples from 900 individuals in nine Local Government Areas in Ebonyi State, Nigeria were examined for Paragonimus ova using concentration technique. The overall prevalence of pulmonary Paragonimus infection in the area was 16.30%. Six foci of the infection were identified in Ebonyi North and Ebonyi Central but none in Ebonyi South. The intensity of the infection was generally moderate. Of the 720 individuals examined, 16 (12.12%) had less than 40 ova of Paragonimus in 5 mL sputum and 114 (86.36%) had between 40 and 79 ova of Paragonimus in 5 mL sputum. -

Rtbfoods Scientific Progress Report for Period 3 (Jan-Dec 2020)
Scientific Progress Report Understanding the Drivers of Trait Preferences and the Development of Multi-user RTB Product Profiles - RTBfoods Scientific Progress Report for Period 3 (Jan-Dec 2020) Montpellier, France, 22 February 2021 Lora FORSYTHE, Natural Resources Institute (NRI), University of Greenwich, Chatham, UK Geneviève FLIEDEL, Centre de coopération Internationale en Recherche Agronomique pour le Développement (CIRAD), Montpellier, France Alexandre BOUNIOL, Université d’Abomey-Calavi, Faculté des Sciences Agronomiques (UAC-FSA) /CIRAD, Cotonou, Benin Tessy MADU, National Root Crops Research Institute (NRCRI), Umudike, Nigeria This report has been written in the framework of RTBfoods project. To be cited as: Lora FORSYTHE, Geneviève FLIEDEL, Alexandre BOUNIOL, Tessy MADU, (2021). Understanding the Drivers of Trait Preferences and the Development of Multi-user RTB Product Profiles – RTBfoods Scientific Progress Report for Period 3 (Jan-Dec 2020). Montpellier, France: RTBfoods Scientific Progress Report, 70 p. Ethics: The activities, which led to the production of this document, were assessed and approved by the CIRAD Ethics Committee (H2020 ethics self-assessment procedure). When relevant, samples were prepared according to good hygiene and manufacturing practices. When external participants were involved in an activity, they were priorly informed about the objective of the activity and explained that their participation was entirely voluntary, that they could stop the interview at any point and that their responses would be anonymous and securely stored by the research team for research purposes. Written consent (signature) was systematically sought from sensory panellists and from consumers participating in activities. Acknowledgments: This work was supported by the RTBfoods project https://rtbfoods.cirad.fr, through a grant OPP1178942: Breeding RTB products for end user preferences (RTBfoods), to the French Agricultural Research Centre for International Development (CIRAD), Montpellier, France, by the Bill & Melinda Gates Foundation (BMGF). -

(GBV) SERVICES REFERRAL DIRECTORY for EBONYI STATE, NIGERIA
GENDER-BASED VIOLENCE (GBV) SERVICES REFERRAL DIRECTORY for EBONYI STATE, NIGERIA Name & Address of Organization Coverage Area Contact Information A HEALTH – Closest referral hospital B PSYCHOSOCIAL COUNSELING Faith Community Counseling Organization (FCCO) Opposite WDC ●Ohaukwu Abakaliki ● Izzi●Ezza Mrs. Margaret Nworie:080-3585-5986 Abakaliki South●Ezza North [email protected] C SHELTER/SAFE HOUSE Safe Motherhood Ladies Association (SMLAS) Mgboejeagu Cresent GRA ●Ohaukwu●Afikpo South●Ezza Mrs. Ugo Ndukwe Uduma: 080-3501-0168 off Ezra Road, Abakaliki South●Abakaliki●Ebonyi●Ohaozara [email protected] Afikpo North●Ishielu Ohaukwu: 070-54178753 Afikpo South: 081-4476-2576 Ezza South: 081-3416-5414 Abakaliki: 070-3838-3692 Izzi: 080-6047-2525 Ebonyi: 080-8877-0802 Ohaozara: 080-6465-2152 Afikpo North: 080-3878-8868; 070-30546995 Ishielu: 080-6838-0889 Family Law Centre No. 47/48, Ezza Road, Abakaliki ●Abakaliki, Ebonyi State Edith Ngene: 080-3416-2207 NSCDC Abakaliki No 8 Town Planning Road P O Box 89 Abakaliki ●Office exists in all LGA Secretariat State Commandant: 080-3669-4912 in the State State PRO: 080-3439-5063 [email protected] NAPTIP Centenary City, 1st Floor Block 10, SMOWAD ●All LGAs Florence Nkechinyere Onwa: 080-3453-4785 D SOCIAL RE-INTEGRATION & ECONOMIC EMPOWERMENT Widow Care Foundation Kilometer 10, Abakaliki-Enugu Expressway ● Abakaliki Mrs. Grace Agbo: 080-3343-1993 Opp. Liberation Estate Abakaliki [email protected] Child Emancipation and Welfare Organization (CEWO) Inside LGA Office, ●Abakaliki Hon. Ishiali Christian: 080-3733-9036 Ohaukwu Catholic Diocese of Abakaliki Succor and Development (SUCCDEV) ●Ohaukwu●Ebonyi●Amaike Aba Sis Cecilia Chukwu: 080-3355-5846 Amaike Aba, Ebonyi LGA, Ebonyi State. -
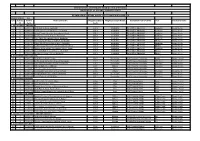
EBONYI STATE 33 S/N S/N for CP Centre Code Name of Centre
SENIOR SCHOOL CERTIFICATE EXAMINATION (EXTERNAL) MASTER LIST OF CENTRES IN EBONYI STATE EXAMINATION CENTRES, CODES AND THEIR NEIGHBOURHOOD EBONYI STATE 33 S/n S/n for Centre Name of Centre Neighbourhood Neighbourhood Name Custodian Point/Outlet LGA Senatorial zone CP Code Code 1. NECO OFFICE ABAKALIKI 1 1 0330001 Girls High School, Abakaliki 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 2 2 0330002 Command Secondary School, Abakaliki 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 3 3 0330003 Bethel Comprehensive Secondary School 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 4 4 0330004 Trinity Secondary School, Abakaliki 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 5 5 0330008 Holy Ghost Secondary School, Abakaliki 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 6 6 0330009 Government Technical College, Abakalilki 3301 Abakaliki Neco Office Abakaliki Ebonyi Ebonyi North 7 7 0330010 Urban Model Secondary School, Abakaliki 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 8 8 0330011 Model Comprehensive Girls Secondary School 3301 Abakaliki Neco Office Abakaliki Ebonyi Ebonyi North 9 9 0330022 Ginger International Secondary School, Abaki 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 10 10 0330023 Abakaliki High School Presco, Abakaliki 3301 Abakaliki Neco Office Abakaliki Ebonyi Ebonyi North 11 11 0330030 Nnodo Secondary School Ababaliki 3301 Abakaliki Neco Office Abakaliki Ebonyi Ebonyi North 4. SUB TREASURY EZZAMGBO 15 1 0330015 Girls High School, -

Linguistic and Geographical Survey of Ebonyi State, South East Nigeria
International Journal of Humanities and Social Science Invention (IJHSSI) ISSN (Online): 2319 – 7722, ISSN (Print): 2319 – 7714 www.ijhssi.org ||Volume 10 Issue 5 Ser. I || May 2021 || PP 01-10 Linguistic and Geographical Survey of Ebonyi State, South East Nigeria 1Evelyn Chinwe Obianika, 1Ngozi U. Emeka-Nwobia, 1Mercy Agha Onu, 1Maudlin A. Eze, 1Iwuchukwu C. Uwaezuoke, 2Okoro S. I., 3Igba D. I., 1Henrietta N. Ajah, 1Emmanuel Nwaoke and Ogbonnaya Joseph Eze 1Department of Linguistics and Literary Studies, Ebonyi State University, Abakaliki. 2Department of History and International Relations, Ebonyi State University, Abakaliki. 3Department of Arts and Social Science Education, Ebonyi State University, Abakaliki. 4Department of Geography, Ebonyi State College of Education, Ikwo Abstract The objective of this study was to identify the major languages spoken in Ebonyi State, their dialects and geographical locations presented in a map. The survey research method was used. Data collection was carried out using personal interview and Focus Group Discussion (FGD) methods. Three men and three female respondents were sampled from each dialect/ethnic group using purposive random sampling and data collection sessions were recorded electronically. The data were analyzed using the descriptive and inferential methods. In the results, two languages were identified in the state; the Igbo and Korin languages. The study found out that the Korin language is spoken in nine major communities and has five identifiable dialects spanning three local government -

Prevalence of Urinary Schistosomiasis Amongst Primary School Children in Ikwo and Ohaukwu Communities of Ebonyi State, Nigeria
African Journal of Laboratory Medicine ISSN: (Online) 2225-2010, (Print) 2225-2002 Page 1 of 5 Original Research Prevalence of urinary schistosomiasis amongst primary school children in Ikwo and Ohaukwu Communities of Ebonyi State, Nigeria Authors: Background: Urinary schistosomiasis is a serious public health challenge in some communities 1 Nse O. Umoh of Ebonyi State, south-east Nigeria, partly resulting from a lack of adequate epidemiological Chimezie F. Nwamini1 Nyoho J. Inyang2 data for the institution of effective control strategies. Anthony N. Umo3 Objective: This study evaluated the prevalence and risk factors of urinary schistosomiasis in Victor U. Usanga1 Amos Nworie1 rural communities of Ebonyi State, south-east Nigeria. Michael O. Elom1 Boniface N. Ukwah1 Methods: A total of 300 students, comprising 185 boys and 115 girls, were randomly selected for the study between July and December 2016. A questionnaire was administered to all Affiliations: participants to determine the risk factors for the disease in the area. Urine specimens collected 1 Department of Medical from the participants were processed by sedimentation and examined microscopically for the Laboratory Science, Faculty of Health Sciences and eggs of Schistosoma haematobium. Technology, Ebonyi State Results: The overall prevalence rate for urinary schistosomiasis was 8.0 . Students aged 6–10 years University, Abakaliki, Nigeria % had the highest prevalence of infection (10.3%). The prevalence was significantly higher amongst 2Department of Medical male students (10.3%; p = 0.038) compared with female students (4.4%). Logistic regression analysis Laboratory Science, Faculty showed a significant association between schistosomiasis infection and freshwater contact activities of Basic Medical Sciences, (p = 0.007; odds ratio = 1.89; 95% confidence interval: 4.33–16.17). -

Ebonyi State Ring Road Project Country
AFRICAN DEVELOPMENT BANK Public Disclosure Authorized PROJECT: EBONYI STATE RING ROAD PROJECT COUNTRY: NIGERIA PROJECT APPRAISAL REPORT Disclosure Authorized Public RDNG/PICU April 2019 TABLE OF CONTENTS I – STRATEGIC THRUST & RATIONALE ....................................................................... 1 1.1. Background ..................................................................................................................... 1 1.2. Project linkages with country Strategy ........................................................................... 1 1.3. Rationale for Bank’s involvement .................................................................................. 2 1.4. Donor coordination ......................................................................................................... 3 II – PROJECT DESCRIPTION ............................................................................................. 4 2.1. Project components ......................................................................................................... 4 2.2. Technical solution retained and other alternatives explored ........................................... 4 2.3. Project type ..................................................................................................................... 5 2.4. Project cost and financing arrangements ........................................................................ 5 2.5. Project’s target area and population ............................................................................... -

ESIA Summaryfinal Draft Jigawa Solar23.5.17.Docx
Language: English Original: English PROJECT: EBONYI STATE RING ROAD COUNTRY: FEDERAL REPUBLIC OF NIGERIA RESETTLEMENT ACTION PLAN (RAP) SUMMARY 28th NOVEMBER 2018 Task Manager: P MUSA, Senior Transport Engineer, RDNG C. MHANGO, Environmental Officer, SNSC 1 RESETTLEMENT ACTION PLAN (RAP) SUMMARY Project Title: EBONYI STATE RING ROAD Project Number: P-NG-DB0-014 Country: FEDERAL REPUBLIC OF NIGERIA Sector: PICU Project Category: 1 Introduction The Ebonyi state government proposes to borrow the total sum of $150 million from the African Development Bank (ADB) and Islamic Development Bank (IsDB) for the reconstruction/ rehabilitation of the 198 km existing Abakaliki Ring Road into a standard one carriageway, 2 - lane road finished with asphalt over lay over the road pavement. The pavement substratum is designed to have a compacted sub soil layer preferably of laterite. This layer is expected to be the stable bed on which the road pavement is carried. The Abakaliki Road Project, otherwise known as the State Ring Road Project, will comprise the upgrading/Rehabilitation of four (4) road links forming the ring road around the Ebonyi state capital; Abakaliki (Map1.1). The circular road is a State road linking series of agrarian communities and villages along Ezzamgbo - Onueke - Noyo - Effium - Ezzamgbo junction road intersect on Abakaliki - Enugu Federal Highway. The road also intersects the Abakaliki - Afikpo - Okigwe Road at Onueke town. Similarly, the road intersects the Abakaliki - Ogoja Federal Highway at Noyo town. The road traverses seven (7) of the thirteen LGAs of the State - Abakaliki, Ebonyi, Ezza South, Ezza North, Ikwo, Izzi and Ohaukwu. Five of the seven local government areas account for 36% of the total land area of the state of which two are largest in the state, in terms of land areas namely: Ikwo and Ohaukwu. -

Mycotoxin Contamination of Herbal Medications on Sale in Ebonyi State, Nigeria
Available online at http://www.ifgdg.org Int. J. Biol. Chem. Sci. 14(2): 613-625, February 2020 ISSN 1997-342X (Online), ISSN 1991-8631 (Print) Original Paper http://ajol.info/index.php/ijbcs http://indexmedicus.afro.who.int Mycotoxin contamination of herbal medications on sale in Ebonyi State, Nigeria Richard C. IKEAGWULONU1*, Charles C. ONYENEKWE1, Nkiruka R. UKIBE1, Charles G. IKIMI2, Friday A. EHIAGHE1, Isaac P. EMEJE1 and Solomon N. UKIBE3 1Department of Medical Laboratory Science, College of Health Sciences, Nnamdi Azikiwe University, Nnewi Campus, P. M. B 5025, Anambra State, Nigeria. 2Department of Biochemistry, Faculty of Science, Federal University of Otuoke, Beyalsa State, Nigeria. 3Department of Microbiology, Faculty of Medicine, Faculty of Medicine, Nnamdi Azikiwe University, Nnewi Campus, P. M. B 5025, Anambra State, Nigeria. *Corresponding author; E-mail: [email protected]. Tel: +23407080013228. ABSTRACT The practice of herbal medication is as old as the culture of the people and despite the advent of modern medication, many people of south eastern Nigeria, still patronizes herbal medication. Herbal medications are consumed directly and could be contaminated with mycotoxins which are detrimental to human and animal health. This study was therefore, designed to determine the extent of mycotoxin contamination of herbal medications on sale in Ebonyi State, South-Eastern Nigeria. In this regard, a multistage random sampling technique was used to select 19 herbal medication samples from stores and markets in Ebonyi State, Nigeria and evaluated for occurrence of three major mycotoxins- aflatoxins (AFs), ochratoxin A (OTA) and fumonisins (FB). Employing wet extraction procedure, mycotoxin occurrence and levels were determined via lateral flow immunoassay technique. -

AGRIC JOR 2021 APR 4.Cdr
N I G E R I A N A G R I C U L T U R A L J O U R N A L ISSN: 0300-368X Volume 52 Number 1, April 2021 Pg. 157-164 Available online at: http://www.ajol.info/index.php/naj https://www.naj.asn.org Creative Commons User License CC:BY ANALYSES OF PRODUCTION COST AND MARKETING OUTLETS FOR SWEETPOTATO FARMERS IN EBONYI STATE NIGERIA Ejechi, M.E. National Root Crops Research Institute, Umudike, PMB 7006, Umuahia, Abia State, Nigeria. Corresponding Authors' email: [email protected] Abstract Despite the growing importance and known potential of sweet potato as food, animal feed and raw material; there is dearth of records of its production and marketing in Nigeria's food system. The study sought to investigate production cost and marketing outlets of sweet potato among farmers in Ebonyi State. Primary data were collected from 400 small-scale sweet potato farmers in the area using a multi-stage sampling technique. The instruments used for data collection were interview schedule and focus group discussion (FGD). Data collected through these methods were analysed using descriptive statistics. FGD was analysed by transcribing responses of the discussants. Findings revealed that land preparation (per ha) constitute the highest cost of production among all the cost items with average cost of ₦25,912.30, also, average total cost of producing one hectare of sweet potato in the study area was ₦71,011.24, while the average sale of harvested produce was ₦249,363.87. This implies an average profit of ₦178,352.1 realized. -

Political Violence and Women Political Participation in Nigeria: a Study of Ebonyi State, 2015 – 2019
International Journal of Business and Management Invention (IJBMI) ISSN (Online): 2319-8028, ISSN (Print):2319-801X www.ijbmi.org || Volume 9 Issue 9 Ser. II || September 2020 || PP 11-22 Political Violence and Women Political Participation in Nigeria: A Study of Ebonyi State, 2015 – 2019 Joy Ucha Egwu Department of Political Science Ebonyi State University, Abakaliki. ABSTRACT This paper, Political Violence and Women Political Participation in Nigeria: a Study of Ebonyi State, 2015 - 2019, focuses on how political violence is a factor for the low participation of women in Nigerian political activities, particularly in Ebonyi State. The study adopted the Radical Feministic theory. The paper establishes that political violence is prevalent in Ebonyi state and it is caused by unemployment, poverty, imposition of candidates among others. It further recognizes the fact that the level of women participation in politics is low owing to ignorance, gender inequality and political violence. It therefore recommends among others that there is need for sustained awareness and value re-orientation campaigns on the importance of politics that is free from violence so that more women can confidently participate in politics and contribute their own quota to national development. --------------------------------------------------------------------------------------------------------------------------------------- Date of Submission: 29-08-2020 Date of Acceptance: 14-09-2020 --------------------------------------------------------------------------------------------------------------------------------------- I. INTRODUCTION Political violence has been part of human history. Many societies have lived with it; unfortunately it affects women‟s participation in leadership circles because women are perceived as not a force to reckon with in politics especially in Nigeria. Females constitute a greater proportion of the population in Nigeria; about 49.36% (World Bank Report, 2012) but, in spite of this, they are not heard.