The 21 Century Electric Car Tesla Motors Energy Efficiency
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Audi Secures Second Starting Position in Qualifying Thriller Quotes After
Portland, 4 August 2001 Audi secures second starting position in qualifying thriller Spectators along Portland International Raceway will find a rather unusual picture on Sunday: For the first time in almost 15 months there is not an Audi at the front of the starting grid. After a qualifying thriller for the American Le Mans Series (ALMS) round at Portland, Rinaldo Capello and Tom Kristensen had to relinquish pole position to the fastest Panoz. Team mates Frank Biela and Emanuele Pirro in the companion Infineon Audi R8 will start the race from third place. During only 20 minutes of qualifying on the circuit situated in the US state of Oregon, the Audi drivers executed exciting duels with a lot of position changes on the time screen. The Audi Sport North America team not only fought against the two Panoz cars, but also against the 2000-spec Champion Audi R8 of Andy Wallace and Johnny Herbert. At the end, the former Formula 1 driver achieved fifth position on the grid. The race starts on Sunday at 13 hours local time and runs over a distance of 2:45 hours. Tom Kristensen (#1) and Frank Biela (#2) will start the race in the two Infineon Audi R8s. Quotes after qualifying Rinaldo Capello (#1): “Concerning our tire choice, we were a little bit conservative in qualifying. We knew we would not be that strong in this session because we wanted to work mainly for the race tomorrow.” Tom Kristensen (#1): “Of course it’s a little bit disappointing to be so close to pole and then have to start only from second position. -

Units and Magnitudes (Lecture Notes)
physics 8.701 topic 2 Frank Wilczek Units and Magnitudes (lecture notes) This lecture has two parts. The first part is mainly a practical guide to the measurement units that dominate the particle physics literature, and culture. The second part is a quasi-philosophical discussion of deep issues around unit systems, including a comparison of atomic, particle ("strong") and Planck units. For a more extended, profound treatment of the second part issues, see arxiv.org/pdf/0708.4361v1.pdf . Because special relativity and quantum mechanics permeate modern particle physics, it is useful to employ units so that c = ħ = 1. In other words, we report velocities as multiples the speed of light c, and actions (or equivalently angular momenta) as multiples of the rationalized Planck's constant ħ, which is the original Planck constant h divided by 2π. 27 August 2013 physics 8.701 topic 2 Frank Wilczek In classical physics one usually keeps separate units for mass, length and time. I invite you to think about why! (I'll give you my take on it later.) To bring out the "dimensional" features of particle physics units without excess baggage, it is helpful to keep track of powers of mass M, length L, and time T without regard to magnitudes, in the form When these are both set equal to 1, the M, L, T system collapses to just one independent dimension. So we can - and usually do - consider everything as having the units of some power of mass. Thus for energy we have while for momentum 27 August 2013 physics 8.701 topic 2 Frank Wilczek and for length so that energy and momentum have the units of mass, while length has the units of inverse mass. -

Panoz Launches New Esperante “Gt” Edition of America’S Premier Hand-Built Aluminum Sports Car
News Release FOR IMMEDIATE RELEASE For More Information Contact Scott Black at TimePiece PR (214) 520-3430 (ext. 303) PANOZ LAUNCHES NEW ESPERANTE “GT” EDITION OF AMERICA’S PREMIER HAND-BUILT ALUMINUM SPORTS CAR New Car To Be Unveiled at LA Auto Show Los Angeles – December 29, 2003 – Company founder and CEO Dan Panoz will unveil a new “GT” edition of the Panoz Esperante sports car at the Los Angles International Auto Show on December 29, 2003. The Esperante GT features the dramatic new body treatment and numerous performance enhancements from the exotic Panoz Esperante GT-LM. Panoz Auto Development, America's premier independent manufacturer of hand built, high performance automobiles, builds Esperante sports and racecars near Atlanta, Georgia. “Upon introduction of the limited edition Esperante GT-LM earlier this year, there were many requests to offer some of the car’s innovations on our standard model,” said Mr. Panoz. “That strong demand, coupled with our commitment to maintain the exclusivity of the exotic GT-LM, led us to develop the Esperante GT. This car offers world-class performance, rarity and a handsome body in a sophisticated, hand-built package. As Southern California is the sports car capital of America, it seemed appropriate to unveil this spectacular new vehicle at the LA Auto Show.” Like the GT-LM, the Esperante GT gives the remarkable Panoz platform a decidedly more sporting tone based on Panoz’s racing experience in the American Le Mans Racing Series. The rear subframe has been redesigned to feature a more aggressive suspension geometry and increase its structural stiffness while simultaneously reducing its weight. -

January 15, 2008
THE BOARD OF SUPERVISORS OF THE COUNTY OF STANISLAUS DEPT: Environmental Resources BOARD AGENDA # *B-8 Urgent Routine AGENDA DATE Janualy 15, 2008 CEO Concurs with Recommendation YES @NO 415Vote Required YES NO (Info ation Attached) SUBJECT: Authorization to Apply for, Administer and Receive Funds for a Waste Tire Enforcement Grant from the California lntegrated Waste Management Board STAFF RECOMMENDATIONS: 1. Adopt a Resolution to authorize the Director of the Department of Environmental Resources, or her designee, to apply for, administer and receive the Fiscal Year 2007-2008 Waste Tire Enforcement Grant TEA-15. 2. Authorize the submittal of a joint funding request to the California Integrated Waste Management Board for a Waste Tire Enforcement Grant TEA-15 on behalf of the cities of Ceres, Hughson, Newman, Oakdale, Patterson, Riverbank, Turlock, Waterford, and the unincorporated areas of Stanislaus County. 3. Direct the Auditor-Controller to increase appropriations and estimated revenue in the amount of $127,829 as detailed in the Budget Journal Form when the grant is awarded. FISCAL IMPACT: This grant would provide approximately $127,829 during Fiscal Year 2008-2009 in reimbursements to the Department of Environmental Resources for expenses to implement a regional waste tire enforcement program for the unincorporated areas of Stanislaus County and the cities of Ceres, Hughson, Newman, Oakdale, Patterson, Riverbank, Turlock and Waterford. The exact amount of the grant is not yet known but is anticipated to not exceed 10% in additional funds or $140,612. If the actual grant amount exceeds $127,829, an adjustment will be made during Final Budget. ......................................................................................................................... BOARD ACTION AS FOLLOWS: ATTEST: CHRISTINE FERRARO TALLMAN, Clerk File No. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -
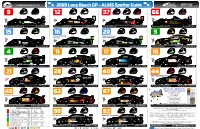
ALMS Spotter Guide
andyblackmoredesign.com 2009 Long Beach GP - ALMS Spotter Guide 9 Brabham Sharp 12 Willman Lewis 37 J.Field C.Field Ducote 66 de Ferran Pagenaud Patron Highcroft Racing Acura ARX-02a Autocon Motorsports Lola B06/10-AER Intersport Racing Lola B06/10-AER de Ferran Motorsports Acura ARX-02a 15 Fernandez Diaz 16 Dyson G.Smith 20 Leitzinger M.Franchitti 3 Magnussen O’Connell Green windshield strip Yellow windshield stripe, and mirrors mirrors and nose tips Lowe’s Fernandez Racing Acura ARX-01b Dyson Racing Team Lola B09/86-Mazda Dyson Racing Team Lola B09/86-Mazda Corvette Racing Chevrolet Corvette C6-R 4 Beretta Gavin 11 Feinberg Hall 17 Sellers Cicero 18 Westbrook J.Stuck www.falkentire.com Corvette Racing Chevrolet Corvette C6-R Primetime Race Group Dodge Viper Coupe Team Falken Tire Porsche 911 GT3-RSR VICI Racing Porsche 911 GT3-RSR 1 1 21 Farnbacher James 28 Gigliotti Said 40 D.Robertson Murry 44 Law Neiman Blue windshield stripe, mirrors Panoz Team PTG Panoz Esperante GTLM-Ford LG Motorsport Chevrolet-Riley Corvette C6 Robertson Racing Doran Ford GT Mk7 Flying Lizard Motorsport Porsche 911 GT3 RSR American Le Mans Series 2008 Season Yearbook Large format 12”x9” 45 Bergmeister Long 62 Melo Kaffer 87 Henzler Werner 260 Pages Yellow windshield stripe, mirrors 900+ Images Full race reports & stats Le Mans 24 Hours Winter test review Chassis & Team sections Techincal details Artwork from Andy Blackmore Design Flying Lizard Motorsport Porsche 911 GT3 RSR Risi Competizione Ferrari 430 GT Farnbacher Loles Porsche 911 GT3 RSR Available from ALMS Website,Vendors or @ www.TPPpublishing.com Points Leaderboard (after 2 Rounds) COPYRIGHT 2009- Produced Exclusively for the partners shown in the header and supported by 1. -

Letters from Cuba
Martina Butler reports on the CLC Winter Board Meeting in Scottsdale - page 7 The Issuetailfin 25 • February 2015 www.peachstateclc.com LETTERS FROM CUBA PG. 14 FIND YOUR CADILLAC’S DATA BOOK! BILL YOUNG’S 1971 ELDORADO DREAM CAR PG. 23 PG. 12 THE TAILFIN is a publication of Peach State CLC, a region of the Cadillac and LaSalle Club, Inc., and is distributed to its members. Prerequisite for membership in Peach State CLC is current or pending membership in the national club. Member costs are $25 a year for Peach State CLC. The CLC, Inc. dues categories may be found at: http;//www.cadillaclasalleclub.org/ P EACH S TATE C ADILLAC & L A S ALLE C LUB F EBRUARY 2 0 1 5 Contents We’re excited that our Peach State web- Valentine’s Day at Château Élan site has been honored with a regional 4 Merit Award! Check out our site at: Grab your sweetheart and join us as we http://peachstateclc.com/ Cruise-on-over to Braselton, GA and enjoy a fabulous lunch! You may even choose to stick around and tour the Château’s President famous winery. Doug Bailey 770-992-8394 H [email protected] 7 CLC Winter Board Meeting [email protected] Martina reports on a busy agenda Vice President in Scottsdale. Read all about ideas Kevin Garrison and upcoming national-sanctioned 706-207-1550 events coming up this year and [email protected] several years into the future. Corresponding Secretary Sandy Barth A ‘54 Eldorado in the UK 770-663-8327 H 14 770-630-2417 C Leigh Spivey weaves an amazing story [email protected] about the great times of his UK-famous Membership Director “KKK” Eldo that’s been featured in Cecil “Buster” Miller commercials, films, parades and 706-517-0601 H even advertising for Kit-Kats! 706-264-9558 W [email protected] Data Books and Historic Treasurer 23 Michelle Anderson Cadillac Literature 706-270-8976 H Wondering where to get a [email protected] Cadillac Build Sheet for your Activities Directors favorite ride? We’ve got the Tom and Linda Di Nucci source link and details. -
![Arxiv:1303.1588V2 [Physics.Hist-Ph] 8 Jun 2015 4.4 Errors in the Article](https://docslib.b-cdn.net/cover/6367/arxiv-1303-1588v2-physics-hist-ph-8-jun-2015-4-4-errors-in-the-article-1106367.webp)
Arxiv:1303.1588V2 [Physics.Hist-Ph] 8 Jun 2015 4.4 Errors in the Article
Translation of an article by Paul Drude in 1904 Translation by A. J. Sederberg1;∗ (pp. 512{519, 560{561), J. Burkhart2;y (pp. 519{527), and F. Apfelbeck3;z (pp. 528{560) Discussion by B. H. McGuyer1;x 1Department of Physics, Princeton University, Princeton, New Jersey 08544, USA 2Department of Physics, Columbia University, 538 West 120th Street, New York, NY 10027-5255, USA June 9, 2015 Abstract English translation of P. Drude, Annalen der Physik 13, 512 (1904), an article by Paul Drude about Tesla transformers and wireless telegraphy. Includes a discussion of the derivation of an equivalent circuit and the prediction of nonreciprocal mutual inductance for Tesla transformers in the article, which is supplementary material for B. McGuyer, PLoS ONE 9, e115397 (2014). Contents 1 Introduction 2 2 Bibliographic information 2 3 Translation 3 I. Definition and Integration of the Differential Equations . .3 Figure 1 . .5 II. The Magnetic Coupling is Very Small . .9 III. Measurement of the Period and the Damping . 12 IV. The Magnetic Coupling is Not Very Small . 19 VII. Application to Wireless Telegraphy . 31 Figure 2 . 32 Figure 3 . 33 Summary of Main Results . 39 4 Discussion 41 4.1 Technical glossary . 41 4.2 Hopkinson's law . 41 4.3 Equivalent circuit parameters from the article . 42 arXiv:1303.1588v2 [physics.hist-ph] 8 Jun 2015 4.4 Errors in the article . 42 4.5 Modifications to match Ls with previous work . 42 ∗Present address: Division of Biological Sciences, University of Chicago, 5812 South Ellis Avenue, Chicago, IL 60637, USA. yPresent address: Department of Microsystems and Informatics, Hochschule Kaiserslautern, Amerikastrasse 1, D-66482 Zweibr¨ucken, Germany. -

From Cell to Battery System in Bevs: Analysis of System Packing Efficiency and Cell Types
Article From Cell to Battery System in BEVs: Analysis of System Packing Efficiency and Cell Types Hendrik Löbberding 1,* , Saskia Wessel 2, Christian Offermanns 1 , Mario Kehrer 1 , Johannes Rother 3, Heiner Heimes 1 and Achim Kampker 1 1 Chair for Production Engineering of E-Mobility Components, RWTH Aachen University, 52064 Aachen, Germany; c.off[email protected] (C.O.); [email protected] (M.K.); [email protected] (H.H.); [email protected] (A.K.) 2 Fraunhofer IPT, 48149 Münster, Germany; [email protected] 3 Faculty of Mechanical Engineering, RWTH Aachen University, 52072 Aachen, Germany; [email protected] * Correspondence: [email protected] Received: 7 November 2020; Accepted: 4 December 2020; Published: 10 December 2020 Abstract: The motivation of this paper is to identify possible directions for future developments in the battery system structure for BEVs to help choosing the right cell for a system. A standard battery system that powers electrified vehicles is composed of many individual battery cells, modules and forms a system. Each of these levels have a natural tendency to have a decreased energy density and specific energy compared to their predecessor. This however, is an important factor for the size of the battery system and ultimately, cost and range of the electric vehicle. This study investigated the trends of 25 commercially available BEVs of the years 2010 to 2019 regarding their change in energy density and specific energy of from cell to module to system. Systems are improving. However, specific energy is improving more than energy density. -

Panoz Esperanteesperante GGTSGTTSS RVP – Reliability / Value / Performance
PanozPanoz EsperanteEsperante GGTSGTTSS RVP – Reliability / Value / Performance Quaker State – Recommended fill for the GTS Welcome to the Panoz family. We want to thank you for all of the time The Power to Reduce Friction that you have taken and for placing your trust in us by purchasing a Panoz Esperante GTS Race Car. We look forward to working and playing with you at the race track. This manual is meant to assist you in the care, tuning and enjoyment of your GTS. The information compiled in this book is from our own experience and that of your fellow racers. This manual will be constantly updated as components are upgraded and information is accumulated. We would also appreciate your input for future revisions to this book. This manual will not provide specific race set-ups and strategies. We will explain how to tune your suspension but other than a neutral starting point we won’t tell you how to set your suspension for individual events. For fur- ther information about race strategies, suspension tuning, car set-ups and driving techniques we recommend the following books: • Tune to Win- Carroll Smith • Drive to Win- Carroll Smith • Prepare to Win- Carroll Smith Taking Delivery We have checked and double-checked the condition of the car. It should be in good condition, however, we strongly suggest going through the car with a torque wrench and a careful eye before you take it to the track. If anything is wrong or out of place please let us know, we want to make sure our system is working. -

Audi in the American Le Mans Series – from Sebring to Adelaide
Audi in the American Le Mans Series – From Sebring to Adelaide The Le Mans 24 Hour race ended with a clear triple victory for Audi in 2000. This classic is not only one of the greatest challenges in international motorsport, it is at the same time one of the most popular sporting events in the world. For two years a new championship has been run specifically for the fascinating Le Mans sportscars: The American Le Mans Series (ALMS). The Team Audi Sport North America competed in the twelve round series for the first time in 2000 – with huge success: Two races before the season final, Audi already clinched both the manufacturers´ and the drivers‘ titles. At nine out of twelve rounds an Audi R8 crossed the finish line first. Audi celebrated six double victories. With a triumph at the season final in Australia’s Adelaide, AUDI AG crowned one of the most successful years of its long motorsport tradition. A summary of the ALMS races: Sebring: Audi takes off with a dream start into the 2000 season. Team Audi Sport North America celebrates a double victory at the 12 hour classic in Florida, despite one of the two R8 losing precious time after a tyre puncture. The third-placed BMW is one lap behind. 1. Biela/Kristensen/Pirro (Audi R8) 360 laps 2. Alboreto/Capello/McNish (Audi R8) + 39.111s 3. Lehto/Müller (BMW) - 1 lap 4. Auberlen/Gounon/Soper (BMW) - 2 laps 5 Lienhard/Theys/Baldi (Doran-Judd) - 23 laps 6. Taylor/Angelelli/van de Poele (Cadillac) - 29 laps Charlotte: As the preparation for the Le Mans 24 Hour race has first priority, Team Audi Sport North America competes in North Carolina with two cars in the 1999-spec (R8R). -

2002 Panoz GTLM Supercharged Proto Type Coupe
hoveymotorcars.com Hovey 210-384-0084 Motorcars 30775 IH-10 West Boerne, Texas 78006 2002 Panoz GTLM Supercharged Proto type Coupe View this car on our website at hoveymotorcars.com/6880596/ebrochure Our Price $0 Specifications: Year: 2002 VIN: 1P9PB48362B213076 Make: Panoz Model/Trim: GTLM Supercharged Proto type Coupe Condition: Pre-Owned Body: Coupe Exterior: Sterling Silver Metallic Engine: 4.6LV8 SuperCharged Interior: Black Leather Leather Transmission: 6 Speed Manual Mileage: 10,877 Drivetrain: Rear Wheel Drive This 2002 Panoz Esperante GTLM Coupe was hand made at the Panoz Factory for Celebrity Patrick Dempsey. This was the first Street Legal Coupe made. The Drivers compartment tub is Carbon Fiber, as well as the prototype top. The carbon fiber is polished in the door seals. This vin on this Panoz is ends in 76, meaning it is the 76th Panoz produced in 2002. The panoz also has a chassis number. In 2002, I was at the Panoz Factory getting our Panoz Certification and met Patrick Dempsey as he was designing his personal Unique Panoz. Hovey personal Unique Panoz. Hovey Motorcars was a Certified Panoz Dealer. This Particular Panoz is extremely rare, as it was the first custom coupe. The other unique thing that separates this coupe from the later ones is the "A" pillar and the top are one piece. The later versions had a seam after the "A" pillar. Most of the the GTLM produced after were all convertibles. Patrick Dempsey was just starting his racing career, and so he wanted this Panoz with more horsepower. He has smaller pulley put on the supercharger for more boost.