Vegetation Differences in Neighboring Old-Growth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

French Broad River Basin Restoration Priorities 2009
French Broad River Basin Restoration Priorities 2009 French Broad River Basin Restoration Priorities 2009 TABLE OF CONTENTS Introduction 1 What is a River Basin Restoration Priority? 1 Criteria for Selecting a Targeted Local Watershed (TLW) 2 French Broad River Basin Overview 3 French Broad River Basin Restoration Goals 5 River Basin and TLW Map 7 Targeted Local Watershed Summary Table 8 Discussion of TLWs in the French Broad River Basin 10 2005 Targeted Local Watersheds Delisted in 2009 40 References 41 For More Information 42 Definitions 43 This document was updated by Andrea Leslie, western watershed planner. Cover Photo: French Broad River, Henderson County during 2004 flood after Hurricanes Frances and Ivan French Broad River Basin Restoration Priorities 2009 1 Introduction This document, prepared by the North Carolina Ecosystem Enhancement Program (EEP), presents a description of Targeted Local Watersheds within the French Broad River Basin. This is an update of a document developed in 2005, the French Broad River Basin Watershed Restoration Plan. The 2005 plan selected twenty-nine watersheds to be targeted for stream, wetland and riparian buffer restoration and protection and watershed planning efforts. This plan retains twenty-seven of these original watersheds, plus presents an additional two Targeted Local Watersheds (TLWs) for the French Broad River Basin. Two 2005 TLWs (East Fork North Toe River and French Broad River and North Toe River/Bear Creek/Grassy Creek) were gardens, Mitchell County not re-targeted in this document due to a re-evaluation of local priorities. This document draws information from the detailed document, French Broad River Basinwide Water Quality Plan—April 2005, which was written by the NC Division of Water Quality (DWQ). -

Tourism Asset Inventory
Asset Asset Management Overview Natural/Scenic Asset Details Cultural/Historic Asset Details Event Asset Details Type: Brief Description Potential Market Draw: Access: Uses: Ownership Supporting Critical Asset is Key Tourism Opportunities are Land Visitor Use Management Interpretation Ranger at Site Visitor Potential Land Protection Species Represents the Type of Cultural Representation has Promotion of event Attendance of Event Event results Event has a NGOs Management marketed through Impact Indicators provided to businesses, Management Policy or Plan Plans Included at Site Facilities at Hazards Status Protection cultural heritage of the Heritage Represented: the support of a is primarily: event is Duration: in increased specific Natural, Cultural, Day Visit, Overnight, 1 = difficult Hiking, Biking, Issues Destination are Being visitors, and community Plan in Place Stakeholder Site Status region diverse group of primarily: overnight marketing Historic, Scenic, Extended 5 = easy Paddling, Marketing Monitored on a members to donate Input Tangible, Intangible, stakeholders Locally, Regionally, One Day, stays in strategy and Event, Educational, Interpretation, Organization / Regular Basis time, money, and/or Both Nationally, Locally, Multiple Days destination economic Informational etc. TDA and Reported to other resources for Internationally, All Regionally, impact TDA asset protection Nationally, indicators Internationally, All Pisgah National Forest Natural Established in 1916 and one of the first national Day Visit, Overnight, 5; PNF in Hiking, Biking, U.S. Federal Pisgah Overcrowding Yes Yes, in multiple ways Nantahalla and y,n - name, year Yes; National At various placs at various At various Any hazard Federally protected See Forest forests in the eastern U.S., Pisgah stretches across Extended Transylvania Rock Climbing, Government Conservancy, at some popular through multiple Pisgah forest Forest listed below locations below locations below associated with public lands for Management several western North Carolina counties. -

Authorize Dan River State Trail
HOUSE BILL 360: Authorize Dan River State Trail. 2021-2022 General Assembly Committee: House Rules, Calendar, and Operations of the Date: April 22, 2021 House Introduced by: Reps. K. Hall, Carter Prepared by: Kellette Wade Analysis of: First Edition Staff Attorney OVERVIEW: House Bill 360 would authorize the Department of Natural and Cultural Resources (Department) to add the Dan River Trail to the State Parks System. CURRENT LAW: The State Parks Act provides that a trail may be added to the State Parks System by the Department upon authorization by an act of the General Assembly. All additions are required to be accompanied by adequate authorization and appropriations for land acquisition, development, and operations. BILL ANALYSIS: House Bill 360 would authorize the Department to add the Dan River Trail to the State Parks System as a State Trail. The use of any segment of the trail crossing property not owned by the Department's Division of Parks and Recreation would be governed by the laws, rules, and policies established by that segment's owner. This addition would be exempt from having to be accompanied by adequate appropriations for land acquisition, development, and operations. Lands needed to complete the trail would be acquired either by donations to the State or by using existing funds in the Land and Water Fund, the Parks and Recreation Trust Fund, the federal Land and Water Conservation Fund, and other available sources of funding. EFFECTIVE DATE: This act would be effective when it becomes law. BACKGROUND: The Dan River is important to North Carolina, flowing 214 miles through Virginia and North Carolina, crossing the state line 8 times. -

2017 High Adventure Program Guide
Camp Daniel Boone Harrison High Adventure Programs 2017 High Adventure Program Guide A leader in high adventure programming since 1978, the Harrison High Adventure Program remains the premier council operated destination for older Scouts, Explorers, and Venture crews in the south-east. We offer activities such as backpacking, rafting, zip-lining, rock climbing, and living history. All treks leaving Camp Daniel Boone are accompanied by a trained staff member. Our guides assist in leading the group through the wilderness, providing necessary first aid, emergency care, and instructing participants in skills essential for navigation and survival in a remote wilderness setting. The patrol method is utilized on all expeditions and leadership development is our goal. Programs are filled on a first-come first-serve basis, so do not delay in making your choice for your high adventure trek. Participants must be at least 13 years of age by June 1, 2017. A completed official BSA Medical Form is required for all High Adventure Programs. Other medical forms will not be accepted. Scouts arriving without the required medical form will be responsible for acquiring a physical, locally, prior to being permitted to begin their trek. Treks will not wait to depart for Scouts without a physical. NOTE: The National Forest Service limits group size to 10 people in a wilderness area. For our backpacking treks this number will include a staff member and one other adult with the crew. (Example: eight Scouts, one adult leader and one trail guide or eight Scouts and two trail guides) Therefore group size is limited to nine participants inclusive of an adult or eight participants without an adult. -

Docket # 2018-318-E - Page 11 of 97
EXHIBIT DJW - 5.0 ELECTRONICALLY Page 1 of 18 Date: May 14, 2015 Document: EXHIBIT 2 – AMENDED STIPULATIONS – PLEA AGREEMENT Cases: US DISTRICT COURT FOR THE EASTERN DISTRICT OF NORTH CAROLINA WESTERN DIVISION NUMBERS 5:15-CR-67-H-2 AND 5:15-CR-68- H-2 FILED Findings: - 2019 1. Dan River Steam Station (pages 43 - 48) – The Court found Defendants guilty and Defendants plead guilty to four counts (sets of violations) at Dan River. March a. Count One is that the company violated the Clean Water Act for the unpermitted discharge through the 48-inch stormwater so and the Defendant aided and abetted another 4 in doing so. Furthermore, the Court found that the Defendant acted negligently in doing 4:55 so. b. Count Two is that Defendant violated the CWA by not maintaining the 48-inch storm PM water pipe which constituted a violation of its NPDES permit which requires that the - permittee to properly maintain its equipment. Furthermore, the Court found that the SCPSC Defendant acted negligently in doing so and that the Defendant aided and abetted another in doing so. c. Count Three is that Defendant violated the CWA for the unpermitted discharge through - the 36-inch stormwater pipe at Dan River of coal ash and coal ash wastewater from a Docket point source into a water of the US. Furthermore, the Court found that the Defendant acted negligently in doing so and that the Defendant aided and abetted another in doing # so. 2018-318-E d. Count Four is that Defendant violated the CWA by not maintaining the 36-inch storm water pipe which constituted a violation of its NPDES permit which requires that the permittee to properly maintain its equipment. -
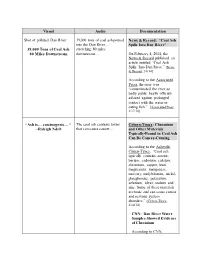
Visual Audio Documentation Shot of Polluted Dan River. 39,000 Tons Of
Visual Audio Documentation Shot of polluted Dan River. 39,000 tons of coal ash poured News & Record: “Coal Ash into the Dan River… Spills Into Dan River” 39,000 Tons of Coal Ash stretching 80 miles 80 Miles Downstream downstream… On February 4, 2014, the News & Record published an article entitled “Coal Ash Spills Into Dan River.” [News & Record, 2/4/14] According to the Associated Press, the river was “contaminated the river so badly public health officials advised against prolonged contact with the water or eating fish.” [Associated Press, 2/17/14] “Ash is… carcinogenic… “ The coal ash contains toxins Citizen-Times: Chromium --Raleigh N&O that can cause cancer… and Other Materials Typically-Found in Coal Ash Can Be Cancer-Causing According to the Asheville Citizen-Times, “Coal ash typically contains arsenic, barium, cadmium, calcium, chromium, copper, lead, magnesium, manganese, mercury, molybdenum, nickel, phosphorous, potassium, selenium, silver, sodium and zinc. Some of these materials are toxic and can cause cancer and nervous system disorders.” [Citizen-Times, 2/10/14] CNN: Dan River Water Samples Showed Evidence of Chromium According to CNN, “Samples taken by the Waterkeeper Alliance last week contained ‘extremely high levels of arsenic, chromium, iron, lead and other toxic metals,’ the group said in a statement.” [CNN, 2/9/14] Chromium is Cancer- Causing According to a Scientific America, “Chromium in Drinking Water Causes Cancer.” From Scientific America: A controversial water contaminant made famous by Erin Brockovich and a small California desert town is carcinogenic. That conclusion by federal scientists, culminating more than a decade of debate, is likely to trigger new, more stringent standards limiting the amount of hexavalent chromium allowable in water supplies. -

H.B. 687 GENERAL ASSEMBLY of NORTH CAROLINA Apr 26, 2021 SESSION 2021 HOUSE PRINCIPAL CLERK H D HOUSE BILL DRH40390-MH-129
H.B. 687 GENERAL ASSEMBLY OF NORTH CAROLINA Apr 26, 2021 SESSION 2021 HOUSE PRINCIPAL CLERK H D HOUSE BILL DRH40390-MH-129 Short Title: Interbasin Transfer Amendments. (Public) Sponsors: Representative Yarborough. Referred to: 1 A BILL TO BE ENTITLED 2 AN ACT TO REMOVE INTRABASIN TRANSFERS AMONG SUBBASINS OF A RIVER 3 BASIN FROM INTERBASIN TRANSFER REQUIREMENTS. 4 The General Assembly of North Carolina enacts: 5 SECTION 1. G.S. 143-215.22G(1b) reads as rewritten: 6 "(1b) "River basin" means any of the following river basins designated on the map 7 entitled "Major River Basins and Sub-basins in North Carolina" and filed in 8 the Office of the Secretary of State on 16 April 1991. means the area within 9 North Carolina denoted by the cataloging unit or series of cataloging units 10 organized by the United States Geologic Survey as designated in this 11 subdivision. The term "river basin" includes any portion of the river basin that 12 extends into another state. Any area outside North Carolina that is not included 13 in one of the river basins listed in this subdivision comprises a separate river 14 basin. 15 a. 1-1 Broad River. 16 b. 2-1 Haw River. 17 c. 2-2 Deep River. 18 d. 2-3 Cape Fear River. 19 e. 2-4 South River. 20 f. 2-5 Northeast Cape Fear River. 21 g. 2-6 New River. 22 h. 3-1 Catawba River. 23 i. 3-2 South Fork Catawba River. 24 j. 4-1 Chowan River. 25 k. -

Water Quality of North Carolina Streams
Water Quality of North Carolina Streams United States Geological Survey Water-Supply Paper 2185-E Prepared in cooperation with the North Carolina Department of Natural and Economic Resources Chapter E Water Quality of North Carolina Streams By DOUGLAS HARMED and DAMN MEYER Prepared in cooperation with the North Carolina Department of Natural and Economic Resources GEOLOGICAL SURVEY WATER-SUPPLY PAPER 2185-E y. s. 9. s. WATER RESOURCES DIVISION ROLLA, MO R E C F ! *< = O JU 2 1983 AM * 7,8I9 iligy^jLj a^M. ft* UNITED STATES DEPARTMENT OF THE INTERIOR JAMES G. WATT, Secretary GEOLOGICAL SURVEY Dallas L. Peck, Director UNITED STATES GOVERNMENT PRINTING OFFICE: 1983 For sale by Distribution Branch Text Products Section U.S. Geological Survey 604 South Pickett Street Alexandria, Virginia 22304 Library of Congress Cataloging in Publication Data Harned, Douglas and Meyer, Dann. Water quality of the Yadkin-Pee Dee River system, North Carolina (Geological Survey water-supply paper ; 2185-E) (Open-file report / U.S. Geological Survey ; 81-643) Bibliography: p. Supt. of Docs, no.: I 19.76:81-643 1. Water quality North Carolina Yadkin River watershed. 2. Water quality Pee Dee River watershed (N.C. and S.C-). I Meyer, Dann. II. North Carolina. Dept. of Natural Resources and Community Development. III. Title. IV. Series. V. Series: Open-file report (United States. Geological Survey); 81-643. TD224.N8H37 553.7'8'0975668 81-607570 AACR2 PREFACE In 1972, the U.S. Geological Survey and the North Carolina Department of Natural Resources and Community Development jointly designed and implemented a statewide monitoring program to help identify current and emerg ing water-quality problems. -

Know Before You Go Guide to the French Broad River and Paddle Trail Headwaters Outfitters Outdoor Adventures
KNOW BEFORE YOU GO GUIDE TO THE FRENCH BROAD RIVER AND PADDLE TRAIL HEADWATERS OUTFITTERS OUTDOOR ADVENTURES • FOUNDED IN 1992 • FAMILY OWNED AND OPERATED FOR 26 YEARS • CANOEING, KAYAKING, AND TUBING ADVENTURES • FLY FISHING ADVENTURES • RETAIL, GEAR, AND FLY SHOP • FORKS OF THE RIVER TAPROOM MOUNTAINTRUE “MOUNTAINTRUE CHAMPIONS RESILIENT FORESTS, CLEAN WATERS, AND HEALTHY COMMUNITIES IN WESTERN NORTH CAROLINA” “Mountaintrue envisions Western North Carolina with thriving communities that are connected to and help sustain a healthy natural environment" • NON-PROFIT ORGANIZATION AND HOME TO THE FRENCH BROAD RIVERKEEPER • CREATED THE PADDLE TRAIL FROM ROSMAN, NC TO DOUGLAS LAKE, TN • ENGAGED IN: • FOSTERING AND EMPOWERING ADVOCATES • POLICY AND PROJECT ADVOCACY • OUTREACH AND EDUCATION • ON THE GROUND PROJECTS RIVERLINK “Promotes the environmental and economic vitality of the French Broad river and its watershed as a place to live, learn, work and play” • CONSERVATION AND IMPROVED WATER QUALITY • EXPANDED EDUCATION AND COMMUNITY AWARENESS • ENHANCED RECREATION OPPORTUNITIES • SUSTAINABLE ECONOMIC DEVELOPMENT • WILMA DYKEMAN RIVERWAY PLAN EXPANSION THROUGHOUT THE WATERSHED HISTORY OF THE FRENCH BROAD RIVER • NAMED BY EUROPEAN SETTLERS IN THE REGION • THIRD OLDEST RIVER IN THE WORLD • NORTH FLOWING RIVER • PREDATES THE UPLIFTING OF THE APPALACHIAN MOUNTAINS • LONGEST FREE-FLOWING RIVER IN NC • 213 MILES FROM ROSMAN, NC TO NEWPORT, TN • CONFLUENCE WITH THE HOLSTEIN RIVER BEGINS THE TENNESSEE RIVER • ENDS IT’S JOURNEY FROM THE MISSISSIPPI RIVER -

Department of Transportation
DocuSign Envelope ID: 1A696EEB-B410-4798-B6DE-41ACBF1B1C9D STATE OF NORTH CAROLINA DEPARTMENT OF TRANSPORTATION ROY COOPER J . Eric Boyette GOVERNOR SECRETARY June 4, 2021 Memorandum To: External Scoping Attendees From: McCray Coates, PE Division Project Manager SUBJECT: External Scoping Meeting Proposed New I-26 Interchange (Future Exit 35), Asheville, Buncombe County NCDOT STIP HE-0001 Division 13 is starting the project development, environmental and engineering studies for the proposed new interchange with I-26 (future exit 35) to access the Pratt & Whitney Manufacturing Center (currently under construction) in Buncombe County. This proposed project includes the construction of a 0.5-1-mile two-lane roadway tie. This proposed roadway tie would connect to the two-lane roadway currently under construction by the private developer which includes a new bridge over the French Broad River and intersects NC 191/Brevard Road. STIP HE-0001 is not included in the current NCDOT 2020-2029 STIP; however, the Department is in the process of having this project programmed. Right of way acquisition and construction let are targeted for Federal Fiscal Year (FFY) 2022 and 2023, respectively. Federal funds will be used for this project and a Type III Categorical Exclusion is anticipated. NCDOT-Division 13 will host a one and one-half hour External Scoping Meeting at 8:30 AM on Wednesday, June 16, 2021. This meeting will be held remotely via a web conference. If you have any questions about the project or the meeting please contact McCray Coates, PE, Division Project Manager, at 828-250-3000 or by email at [email protected]. -

2: Our Home Page 9 2040 Metropolitan Transportation Plan
2: Our Home Page 9 2040 Metropolitan Transportation Plan Introduction Environment Table 2.1: Endangered Species Developing a comprehensive and The Land of Sky region is in the heart of effective transportation plan demands the Southern Appalachians. The forests Flying Squirrel an understanding of the region’s context: and rivers of these ancient mountains the people, goods, communities, and sustain the region’s economy and culture. Peregrine Falcon environment that make up the region and Forests of dogwood, birch, hemlock, Rock Shrew its character. Understanding these traits poplar, maple, oak and pine lead to can help to illustrate the challenges and higher elevation stands of balsam firs Longtail Salamander opportunities in developing transportation and red and black spruce. In the valleys, Slippershell Mussel infrastructure in our region and can help rich soils support a variety of agricultural planners and designers develop solutions crops. In total there are 186,079 acres of Mountain Brook Lamprey for a more effective transportation prime farmland in the study area and over Loggerhead Musk Turtle network. 300,000 acres of working farms and forests. Logperch Western North Carolina is well-known for The region is home to the headwaters its mountain scenery, fast-flowing rivers, of many major river systems. There are Striped Shiner and opportunities for recreation that over 7,688 miles of streams in the region Appalachian Elktoe Mussel make it a major tourist destination in North including 98 square miles of Outstanding Carolina. But many of the elements that Resource Waters, 192 square miles of High Stonecat make our region so attractive also pose Quality Waters, and 246 square miles of Bog Turtle many challenges for developing a safe Water Supply Watersheds. -

The High Peaks and Asheville—MST Segment 3
1 | Mountains-to-Sea Trail Last Updated 1/1/2017 Approaching Blackstock Knob Photo by Jeff Clark www.internetbrothers.org The High Peaks and Asheville—MST Segment 3 PISGAH INN TO BLACK MOUNTAIN CAMPGROUND By Jim Grode Segment 3 is where Asheville comes to play. It is the most heavily used part of the MST within the mountains, and you are likely to encounter hikers, dog walkers, and trail runners at any time throughout the year. Virtually all of it is easily accessible from the Blue Ridge Parkway (BRP), and there are road crossings every few miles for most of the length. Don’t let its accessibility and popularity fool you, however: With eleva- tions ranging from 2,000 feet at the French Broad River to over 6,600 feet at the summit of Mount Mitchell, this segment is quite rugged. Indeed, the Segment 3 | 2 section just west of Asheville hosts the infamous Shut-In Ridge Trail Run, an 18-mile trail run that annually humbles racers from around the country. Complementing the natural beauty of the Blue Ridge Mountains in this area is the vibrancy of Asheville, a city of 80,000 nestled in the French Broad River valley, which regularly makes lists of the top 10 cities in the United States. Crammed with restaurants, shops, art galleries, and brew- eries, Asheville offers something for nearly everyone and is well worth a layover in your hiking schedule. HIGHLIGHTS INCLUDE • The views atop 6,684-foot Mount Mitchell, the highest point east of the Mississippi River • The Shut-In Trail, which follows the old carriage road from the Biltmore House to George Vanderbilt’s hunting lodge on Mount Pisgah (which no longer stands, but a few remnants of which are still visible) • The cultural and scientific displays at the Blue Ridge Parkway Visitor Center & Headquarters near Asheville • The fine collection of southern art and crafts at the Folk Art Center also near Asheville.