Mustang Presentation July
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cancer-Associated Fibroblasts Promote Prostate Tumor Growth And
Neuwirt et al. Cell Communication and Signaling (2020) 18:11 https://doi.org/10.1186/s12964-019-0505-5 RESEARCH Open Access Cancer-associated fibroblasts promote prostate tumor growth and progression through upregulation of cholesterol and steroid biosynthesis Hannes Neuwirt1, Jan Bouchal2, Gvantsa Kharaishvili2, Christian Ploner3, Karin Jöhrer4,5, Florian Pitterl6, Anja Weber7, Helmut Klocker7 and Iris E. Eder7* Abstract Background: Androgen receptor targeted therapies have emerged as an effective tool to manage advanced prostate cancer (PCa). Nevertheless, frequent occurrence of therapy resistance represents a major challenge in the clinical management of patients, also because the molecular mechanisms behind therapy resistance are not yet fully understood. In the present study, we therefore aimed to identify novel targets to intervene with therapy resistance using gene expression analysis of PCa co-culture spheroids where PCa cells are grown in the presence of cancer-associated fibroblasts (CAFs) and which have been previously shown to be a reliable model for antiandrogen resistance. Methods: Gene expression changes of co-culture spheroids (LNCaP and DuCaP seeded together with CAFs) were identified by Illumina microarray profiling. Real-time PCR, Western blotting, immunohistochemistry and cell viability assays in 2D and 3D culture were performed to validate the expression of selected targets in vitro and in vivo. Cytokine profiling was conducted to analyze CAF-conditioned medium. Results: Gene expression analysis of co-culture spheroids revealed that CAFs induced a significant upregulation of cholesterol and steroid biosynthesis pathways in PCa cells. Cytokine profiling revealed high amounts of pro- inflammatory, pro-migratory and pro-angiogenic factors in the CAF supernatant. In particular, two genes, 3-hydroxy- 3-methylglutaryl-Coenzyme A synthase 2 (HMGCS2) and aldo-keto reductase family 1 member C3 (AKR1C3), were significantly upregulated in PCa cells upon co-culture with CAFs. -

Intra- and Inter-Cellular Rewiring of the Human Colon During Ulcerative Colitis
Intra- and Inter-cellular Rewiring of the Human Colon during Ulcerative Colitis The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Smillie, Christopher S. et al. “Intra- and Inter-cellular Rewiring of the Human Colon during Ulcerative Colitis.” Cell, 178, 3 (July 2019): 714–730.e22 © 2019 The Author(s) As Published 10.1016/J.CELL.2019.06.029 Publisher Elsevier BV Version Author's final manuscript Citable link https://hdl.handle.net/1721.1/126822 Terms of Use Creative Commons Attribution-NonCommercial-NoDerivs License Detailed Terms http://creativecommons.org/licenses/by-nc-nd/4.0/ HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Cell. Author Manuscript Author manuscript; Manuscript Author available in PMC 2020 July 25. Published in final edited form as: Cell. 2019 July 25; 178(3): 714–730.e22. doi:10.1016/j.cell.2019.06.029. Cellular and inter-cellular rewiring of the human colon during ulcerative colitis Christopher S. Smillie1,19, Moshe Biton1,2,19, José Ordovas-Montañes1,3,4,5,6,7,19, Keri M. Sullivan8, Grace Burgin1, Daniel B. Graham2,8,9,10,11, Rebecca H. Herbst1,12, Noga Rogel1, Michal Slyper1, Julia Waldman1, Malika Sud1, Elizabeth Andrews8, Gabriella Velonias8, Adam L. Haber1, Karthik Jagadeesh1, Sanja Vickovic1, Junmei Yao14, Christine Stevens9, Danielle Dionne1, Lan T. Nguyen1, Alexandra-Chloé Villani1,13, Matan Hofree1, Elizabeth A. Creasey14, Hailiang Huang15,16, Orit Rozenblatt-Rosen1, John J. Garber8, Hamed Khalili8, A. Nicole Desch9,14, Mark J. Daly15,16,17, Ashwin N. Ananthakrishnan8,*, Alex K. Shalek1,3,4,5,6,*, Ramnik J. -

( 12 ) United States Patent ( 10 ) Patent No .: US 10,837,019 B2 Kochenderfer ( 45 ) Date of Patent : * Nov
US010837019B2 ( 12 ) United States Patent ( 10 ) Patent No .: US 10,837,019 B2 Kochenderfer ( 45 ) Date of Patent : * Nov . 17 , 2020 ( 54 ) CHIMERIC ANTIGEN RECEPTORS ( 56 ) References Cited TARGETING B - CELL MATURATION ANTIGEN U.S. PATENT DOCUMENTS 4,235,871 A 11/1980 Papahadjopoulos et al . ( 71 ) Applicant: The United States of America , as 4,501,728 A 2/1985 Geho et al . represented by the Secretary, 4,837,028 A 6/1989 Allen 5,019,369 A 5/1991 Presant et al . Department of Health and Human 5,087,616 A 2/1992 Myers et al . Services, Bethesda, MD (US ) 5,122,464 A 6/1992 Wilson et al . 5,464,758 A 11/1995 Gossen et al . ( 72 ) Inventor: James N. Kochenderfer , Bethesda , 5,770,359 A 6/1998 Wilson et al . 5,814,618 A 9/1998 Bujard et al. MD ( US ) 7,112,715 B2 9/2006 Chambon et al . 9,765,342 B2 9/2017 Kochenderfer ( 73 ) Assignee : The United States of America , as 2007/0009518 A1 1/2007 Novobrantseva et al . represented by the Secretary, 2009/0093024 A1 4/2009 Bowers et al . Department of Health and Human 2011/0020343 Al 1/2011 Senter et al . 2011/0135639 Al 6/2011 Yu et al . Services , Bethesda, MD (US ) 2012/0148552 A1 6/2012 Jensen 2013/0280221 A1 10/2013 Schonfeld et al . ( * ) Notice : Subject to any disclaimer , the term of this 2013/0287748 A1 10/2013 June et al . patent is extended or adjusted under 35 2018/0051292 A1 * 2/2018 Kochenderfer .. CO7K 14/70517 U.S.C. -

The Interleukin 22 Pathway Interacts with Mutant KRAS to Promote Poor Prognosis in Colon Cancer
Author Manuscript Published OnlineFirst on May 19, 2020; DOI: 10.1158/1078-0432.CCR-19-1086 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. The interleukin 22 pathway interacts with mutant KRAS to promote poor prognosis in colon cancer Authors: Sarah McCuaig1, David Barras,2, Elizabeth Mann1, Matthias Friedrich1, Samuel Bullers1, Alina Janney1, Lucy C. Garner1, Enric Domingo3, Viktor Hendrik Koelzer3,4,5, Mauro Delorenzi2,6,7, Sabine Tejpar8, Timothy Maughan9, Nathaniel R. West1, Fiona Powrie1 Affiliations: 1 Kennedy Institute of Rheumatology, University of Oxford, Oxford UK. 2 SIB Swiss Institute of Bioinformatics, Bioinformatics Core Facility, Lausanne, Switzerland. 3Department of Oncology, University of Oxford, Oxford UK. 4Nuffield Department of Medicine, University of Oxford, Oxford UK. 5Department of Pathology and Molecular Pathology, University and University Hospital Zurich, Zurich Switzerland. 6 Ludwig Center for Cancer Research, University of Lausanne, Lausanne, Switzerland. 7 Department of Oncology, Faculty of Biology and Medicine, University of Lausanne, Lausanne Switzerland. 8 Molecular Digestive Oncology, KU Leuven, Belgium. 9 CRUK/MRC Oxford Institute for Radiation Oncology, University of Oxford, Oxford, UK. Downloaded from clincancerres.aacrjournals.org on September 26, 2021. © 2020 American Association for Cancer Research. Author Manuscript Published OnlineFirst on May 19, 2020; DOI: 10.1158/1078-0432.CCR-19-1086 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Correspondence to: Professor Fiona Powrie; Kennedy Institute of Rheumatology, University of Oxford, Roosevelt Drive, Headington, Oxford, OX3 7YF, UK. Email: [email protected] Conflicts of Interest: S.M., N.R.W., and F.P. -

Modulation of the IL-33/IL-13 Axis in Obesity by IL-13Rα2
Modulation of the IL-33/IL-13 Axis in Obesity by IL-13R α2 Jennifer Duffen, Melvin Zhang, Katherine Masek-Hammerman, Angela Nunez, Agnes Brennan, Jessica This information is current as E. C. Jones, Jeffrey Morin, Karl Nocka and Marion Kasaian of September 29, 2021. J Immunol published online 5 January 2018 http://www.jimmunol.org/content/early/2018/01/05/jimmun ol.1701256 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2018/01/05/jimmunol.170125 Material 6.DCSupplemental http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 29, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2018 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published January 5, 2018, doi:10.4049/jimmunol.1701256 The Journal of Immunology Modulation of the IL-33/IL-13 Axis in Obesity by IL-13Ra2 Jennifer Duffen,* Melvin Zhang,* Katherine Masek-Hammerman,† Angela Nunez,‡ Agnes Brennan,* Jessica E. C. Jones,x Jeffrey Morin,‡ Karl Nocka,* and Marion Kasaian* In obesity, IL-13 overcomes insulin resistance by promoting anti-inflammatory macrophage differentiation in adipose tissue. -

Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association Between BMI and Adult-Onset Non- Atopic
Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association between BMI and Adult-Onset Non- Atopic Asthma Ayoung Jeong 1,2, Medea Imboden 1,2, Akram Ghantous 3, Alexei Novoloaca 3, Anne-Elie Carsin 4,5,6, Manolis Kogevinas 4,5,6, Christian Schindler 1,2, Gianfranco Lovison 7, Zdenko Herceg 3, Cyrille Cuenin 3, Roel Vermeulen 8, Deborah Jarvis 9, André F. S. Amaral 9, Florian Kronenberg 10, Paolo Vineis 11,12 and Nicole Probst-Hensch 1,2,* 1 Swiss Tropical and Public Health Institute, 4051 Basel, Switzerland; [email protected] (A.J.); [email protected] (M.I.); [email protected] (C.S.) 2 Department of Public Health, University of Basel, 4001 Basel, Switzerland 3 International Agency for Research on Cancer, 69372 Lyon, France; [email protected] (A.G.); [email protected] (A.N.); [email protected] (Z.H.); [email protected] (C.C.) 4 ISGlobal, Barcelona Institute for Global Health, 08003 Barcelona, Spain; [email protected] (A.-E.C.); [email protected] (M.K.) 5 Universitat Pompeu Fabra (UPF), 08002 Barcelona, Spain 6 CIBER Epidemiología y Salud Pública (CIBERESP), 08005 Barcelona, Spain 7 Department of Economics, Business and Statistics, University of Palermo, 90128 Palermo, Italy; [email protected] 8 Environmental Epidemiology Division, Utrecht University, Institute for Risk Assessment Sciences, 3584CM Utrecht, Netherlands; [email protected] 9 Population Health and Occupational Disease, National Heart and Lung Institute, Imperial College, SW3 6LR London, UK; [email protected] (D.J.); [email protected] (A.F.S.A.) 10 Division of Genetic Epidemiology, Medical University of Innsbruck, 6020 Innsbruck, Austria; [email protected] 11 MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, W2 1PG London, UK; [email protected] 12 Italian Institute for Genomic Medicine (IIGM), 10126 Turin, Italy * Correspondence: [email protected]; Tel.: +41-61-284-8378 Int. -

Conserved Transcriptomic Profile Between Mouse and Human Colitis
ARTICLE https://doi.org/10.1038/s41467-019-10769-x OPEN Conserved transcriptomic profile between mouse and human colitis allows unsupervised patient stratification Paulo Czarnewski1, Sara M. Parigi1, Chiara Sorini 1, Oscar E. Diaz 1, Srustidhar Das1, Nicola Gagliani1,2,3 & Eduardo J. Villablanca 1,3 1234567890():,; Clinical manifestations and response to therapies in ulcerative colitis (UC) are hetero- geneous, yet patient classification criteria for tailored therapies are currently lacking. Here, we present an unsupervised molecular classification of UC patients, concordant with response to therapy in independent retrospective cohorts. We show that classical clustering of UC patient tissue transcriptomic data sets does not identify clinically relevant profiles, likely due to associated covariates. To overcome this, we compare cross-sectional human data sets with a newly generated longitudinal transcriptome profile of murine DSS-induced colitis. We show that the majority of colitis risk-associated gene expression peaks during the inflammatory rather than the recovery phase. Moreover, we achieve UC patient clustering into two distinct transcriptomic profiles, differing in neutrophil-related gene activation. Notably, 87% of patients in UC1 cluster are unresponsive to two most widely used biological therapies. These results demonstrate that cross-species comparison enables stratification of patients undistinguishable by other molecular approaches. 1 Immunology and Allergy Unit, Department of Medicine, Solna, Karolinska Institute and University Hospital, 17176 Stockholm, Sweden. 2 Department of Medicine and Department of General, Visceral and Thoracic Surgery, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany. 3These authors jointly supervised this work: Nicola Gagliani, Eduardo J. Villablanca. Correspondence and requests for materials should be addressed to E.J.V. -

Strategies to Address Chimeric Antigen Receptor Tonic Signaling Adam Ajina1,2 and John Maher1,2,3,4
Review Molecular Cancer Therapeutics Strategies to Address Chimeric Antigen Receptor Tonic Signaling Adam Ajina1,2 and John Maher1,2,3,4 Abstract Adoptive cell transfer using chimeric antigen receptors Here, we review the mechanisms underpinning CAR tonic (CAR) has emerged as one of the most promising new ther- signaling and highlight the wide variety of effects that can apeutic modalities for patients with relapsed or refractory B- emerge after making subtle structural changes or altering the cell malignancies. Thus far, results in patients with advanced methodology of CAR transduction. We highlight strategies to solid tumors have proven disappointing. Constitutive tonic prevent unconstrained tonic signaling and address its delete- signaling in the absence of ligand is an increasingly recognized rious consequences. We also frame this phenomenon in the complication when deploying these synthetic fusion receptors context of endogenous TCR tonic signaling, which has been and can be a cause of poor antitumor efficacy, impaired shown to regulate peripheral tolerance, facilitate the targeting survival, and reduced persistence in vivo. In parallel, ligand- of foreign antigens, and suggest opportunities to coopt ligand- dependent tonic signaling can mediate toxicity and promote T- dependent CAR tonic signaling to facilitate in vivo persistence cell anergy, exhaustion, and activation-induced cell death. and efficacy. Mol Cancer Ther; 17(9); 1795–815. Ó2018 AACR. Background subtle differences in CAR design can have amplified effects both in vitro and particularly in vivo and that the optimal selection of the Adoptive cell transfer (ACT), utilizing autologous T cells engi- CAR's extracellular targeting moiety, hinge, spacer, transmem- neered to express chimeric antigen receptors (CAR), has proven to brane domain (TMD), and intracellular costimulatory domain(s) be a highly efficacious strategy for the management of patients (ICD) is crucial. -

The Interleukin 22 Pathway Interacts with Mutant KRAS to Promote Poor Prognosis in Colon Cancer
Author Manuscript Published OnlineFirst on May 19, 2020; DOI: 10.1158/1078-0432.CCR-19-1086 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. The interleukin 22 pathway interacts with mutant KRAS to promote poor prognosis in colon cancer Authors: Sarah McCuaig1, David Barras,2, Elizabeth Mann1, Matthias Friedrich1, Samuel Bullers1, Alina Janney1, Lucy C. Garner1, Enric Domingo3, Viktor Hendrik Koelzer3,4,5, Mauro Delorenzi2,6,7, Sabine Tejpar8, Timothy Maughan9, Nathaniel R. West1, Fiona Powrie1 Affiliations: 1 Kennedy Institute of Rheumatology, University of Oxford, Oxford UK. 2 SIB Swiss Institute of Bioinformatics, Bioinformatics Core Facility, Lausanne, Switzerland. 3Department of Oncology, University of Oxford, Oxford UK. 4Nuffield Department of Medicine, University of Oxford, Oxford UK. 5Department of Pathology and Molecular Pathology, University and University Hospital Zurich, Zurich Switzerland. 6 Ludwig Center for Cancer Research, University of Lausanne, Lausanne, Switzerland. 7 Department of Oncology, Faculty of Biology and Medicine, University of Lausanne, Lausanne Switzerland. 8 Molecular Digestive Oncology, KU Leuven, Belgium. 9 CRUK/MRC Oxford Institute for Radiation Oncology, University of Oxford, Oxford, UK. Downloaded from clincancerres.aacrjournals.org on September 30, 2021. © 2020 American Association for Cancer Research. Author Manuscript Published OnlineFirst on May 19, 2020; DOI: 10.1158/1078-0432.CCR-19-1086 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Correspondence to: Professor Fiona Powrie; Kennedy Institute of Rheumatology, University of Oxford, Roosevelt Drive, Headington, Oxford, OX3 7YF, UK. Email: [email protected] Conflicts of Interest: S.M., N.R.W., and F.P. -

Expression of Interleukin-13 Receptor A2 in Glioblastoma Multiforme: Implications for Targeted Therapies
Priority Report Expression of Interleukin-13 Receptor A2 in Glioblastoma Multiforme: Implications for Targeted Therapies John S. Jarboe,1 Kory R. Johnson,2 Yong Choi,1 Russell R. Lonser,3 and John K. Park1 1Surgical and Molecular Neuro-oncology Unit, 2Bioinformatics Group, Intramural Information Technology Program, Division of Intramural Research, and 3Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, NIH, Bethesda, Maryland Abstract quantities of IL4, an effect referred to as IL4-independent IL13 Glioblastoma multiforme is the most common primary binding (2–5). The cell surface expression of a monomeric 42-kDa malignant brain tumor and despite treatment with surgery, receptor capable of binding IL13 but not IL4, termed IL13 receptor a2 (IL13Ra2), was used to explain this effect (6). It was also initially radiation, and chemotherapy, the median survival of patients a with glioblastoma multiforme is f1 year. Glioblastoma multi- reported that significant IL13R 2 expression, as assessed by Northern blotting, occurs exclusively in the testes and in malignant forme explants and cell lines have been reported to over- a express the interleukin-13 receptor A2 subunit (IL13RA2) glioma tissues (7). The putative relative restriction of IL13R 2 relative to nonneoplastic brain. Based on this finding, a expression to glioma cells was subsequently used as a rationale for recombinant cytotoxin composed of IL13 ligand and a trun- the development of several IL13-based treatment strategies including a recombinant cytotoxin composed of IL13 and a cated form of Pseudomonas aeruginosa exotoxin A (IL13- PE38QQR) was developed for the targeted treatment of truncated form of Pseudomonas aeruginosa exotoxin A (IL13- glioblastoma multiforme tumors. -
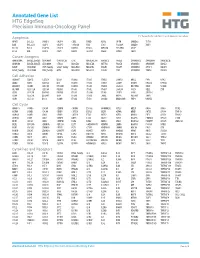
Annotated Gene List HTG Edgeseq Precision Immuno-Oncology Panel
Annotated Gene List HTG EdgeSeq Precision Immuno-Oncology Panel For Research Use Only. Not for use in diagnostic procedures. Apoptosis APAF1 BCL2L1 CARD11 CASP4 CD5L FADD KSR2 OPTN SAMD12 TCF19 BAX BCL2L11 CASP1 CASP5 CORO1A FAS LRG1 PLA2G6 SAMD9 XAF1 BCL10 BCL6 CASP10 CASP8 DAPK2 FASLG MECOM PYCARD SPOP BCL2 BID CASP3 CAV1 DAPL1 GLIPR1 MELK RIPK2 TBK1 Cancer Antigens ANKRD30A BAGE2_BAGE3 CEACAM6 CTAG1A_1B LIPE MAGEA3_A6 MAGEC2 PAGE3 SPANXACD SPANXN4 XAGE1B_1E ARMCX6 BAGE4_BAGE5 CEACAM8 CTAG2 MAGEA1 MAGEA4 MTFR2 PAGE4 SPANXB1 SPANXN5 XAGE2 BAGE CEACAM1 CT45_family GAGE_family MAGEA10 MAGEB2 PAGE1 PAGE5 SPANXN1 SYCP1 XAGE3 BAGE_family CEACAM5 CT47_family HPN MAGEA12 MAGEC1 PAGE2 PBK SPANXN3 TEX14 XAGE5 Cell Adhesion ADAM17 CDH15 CLEC5A DSG3 ICAM2 ITGA5 ITGB2 LAMC3 MBL2 PVR UPK2 ADD2 CDH5 CLEC6A DST ICAM3 ITGA6 ITGB3 LAMP1 MTDH RRAS2 UPK3A ADGRE5 CLDN3 CLEC7A EPCAM ICAM4 ITGAE ITGB4 LGALS1 NECTIN2 SELE VCAM1 ALCAM CLEC12A CLEC9A FBLN1 ITGA1 ITGAL ITGB7 LGALS3 OCLN SELL ZYX CD63 CLEC2B DIAPH3 FXYD5 ITGA2 ITGAM ITLN2 LYVE1 OLR1 SELPLG CD99 CLEC4A DLGAP5 IBSP ITGA3 ITGAX JAML M6PR PECAM1 THY1 CDH1 CLEC4C DSC3 ICAM1 ITGA4 ITGB1 L1CAM MADCAM1 PKP1 UNC5D Cell Cycle ANAPC1 CCND3 CDCA5 CENPH CNNM1 ESCO2 HORMAD2 KIF2C MELK ORC6 SKA3 TPX2 ASPM CCNE1 CDCA8 CENPI CNTLN ESPL1 IKZF1 KIF4A MND1 PATZ1 SP100 TRIP13 AURKA CCNE2 CDK1 CENPL CNTLN ETS1 IKZF2 KIF5C MYBL2 PIF1 SP110 TROAP AURKB CCNF CDK4 CENPU DBF4 ETS2 IKZF3 KIFC1 NCAPG PIMREG SPC24 TUBB BEX1 CDC20 CDK6 CENPW E2F2 EZH2 IKZF4 KNL1 NCAPG2 PKMYT1 SPC25 ZWILCH BEX2 CDC25A CDKN1A CEP250 E2F7 GADD45GIP1 -

Multi-Specific CAR Targeting to Prevent Antigen Escape
Current Hematologic Malignancy Reports (2019) 14:451–459 https://doi.org/10.1007/s11899-019-00537-5 CART AND IMMUNOTHERAPY (M RUELLA AND P HANLEY, SECTION EDITORS) Multi-Specific CAR Targeting to Prevent Antigen Escape Zachary Walsh1 & Savannah Ross1 & Terry J. Fry1 Published online: 22 July 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review Chimeric antigen receptor (CAR) T cell therapy has demonstrated remarkable remission induction rates for relapsed/refractory B cell malignancies. However, loss of the CAR-targeted antigen, known as antigen escape, accounts for a substantial percentage of relapses following CAR therapy and is a major barrier to durable remission. Here, we discuss mech- anisms for antigen escape and strategies to prevent this pattern of relapse, including the use of multi-specific CARs, which recognize and target multiple tumor-associated antigens simultaneously. Recent Findings Preclinical and early clinical trial data indicates that multi-specific CAR therapy for B cell malignancies is both safe and effective. Optimal combinations of target antigens, as well as different multi-specific CAR formats, are currently being evaluated. Summary Although still in early stages of development, multi-specific CAR therapy represents a promising approach to mitigate antigen loss–related relapses and improve durability of remission in patients with refractory B cell malignancies, and may be applicable to other types of cancer. Keywords CAR Tcell . Antigen loss . Antigen escape . Multi-specific . Bivalent . Lineage switch Introduction adult and pediatric relapsed and refractory (r/r) B cell acute lymphoblastic leukemia (B-ALL) (CD19 CAR, 70–90% com- Chimeric antigen receptor (CAR) T cell therapy is a rapidly plete remission (CR) at 28 days), non-Hodgkin’slymphoma evolving immunotherapeutic treatment modality for adult and (NHL) (CD19 CAR, 54–74% CR at 3 months), and multiple pediatric patients with cancer.