Pressure Reducing Support Surfaces - Group 3 (L33692)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Online Driver Training Must Be Completed
DRIVERS SCHOOL/PDX/TES ENDURO RACES July 18-19, 2015 SEBRING INTERNATIONAL RACEWAY 15-DS-3498-S/15-E-3499-S/15-PDX-3500-S Chief Steward. .................................................John Anderson Event Chair ............................................Hollye LaPlante Chief Steward - PDX.......................................Art Trier, Steve Martin Registrar.…………………......………….Robin Ragaglia Asst. Chief Steward - Safety...........................Joe Gandy Safety Scrutineer ...................................Paula Hildock Asst. Chief Steward………………………….....Martyn Eastwood Timing & Scoring ...................................Janet Harhay Asst. Chief Steward .........................................Leland Miller Grid Marshal ..........................................Tim Murphy Asst. Chief Steward ........................................Doug Puckett Pit Marshal.............................................Jay Strole . Starter....................................................Dave MacGregor Chairman S.O.M ……………………………....Krys Dean Sound Control........................................Hollye LaPlante Steward of the Meet …………………………..Norm Esau Pace Car………………………………....Jack Ragaglia Steward of the Meet .......................................Sandy Jung Flagging & Communications.................Joyce Bakels . Paddock Marshal...................................Charlie Leonard . Medical Dir./Course Marshal .................Dave Langston Chief Driver Instructor (School).......................Andy Fox Chief Driver Instructor (PDX)…………...Chris Wells PDX -

2021 Motorsport Australia Manual
2021 MOTORSPORT AUSTRALIA MANUAL RALLY / ROAD APPENDIX GROUP 3C – PRODUCTION RALLY CARS (PRC) motorsport.org.au Modified Article Date of Application Date of Publication 5.2 INDUCTION 01/01/2021 27/11/2020 5.3 SUPERCHARGER 01/01/2021 27/11/2020 5.4 EXHAUST 01/01/2021 27/11/2020 Engine Substitution – approved in 2021 01/02/2021 06/04/2021 Change of NRC references to NRSR Immediate 06/04/2021 Any HEADING is for reference only and has no regulatory effect; and A capitalised and italicised word in this document is defined in the Code or NCR or this document. Production Rally Cars (PRC) are eligible subject to the Automobile meeting the description below and the requirements of Motorsport Australia Manual Schedule A and the National Rally Standing Regulations (NRSR) Vehicles General (VG). NOTE: For Events listed on the FIA International Sporting Calendar, the relevant FIA regulations will apply. 1. GENERAL 1.1 ELIGIBLE AUTOMOBILES (a) Any Automobile, which has a minimum seating capacity of two persons, of which more than 500 have been produced worldwide in the same specification; or (b) An Automobile recognised by the FIA as being of Group A, Group N or Group R (except as shown below) from 1 January 1982; or (c) An Automobile previously recognised by the FIA as being of Group 1 or Group 3 which is included on lists published prior to 31 December 1981; or (d) Any other Automobile being of a model which Motorsport Australia in its sole discretion may recognise for PRC irrespective of the number produced or of its origin. -

Vrg Race Schedule
WATKINS GLEN 2018 WWW.VRGONLINE.ORG Visit the VRG website at www.vrgonline.org for changes and updates to the schedule. VRG RACE SCHEDULE 2018 April 6-8 Wild Hare Run with VDCA – Virginia International Raceway, Alton, VA Royale Formula Ford Feature Race Event Chairman: Mike Jackson, Tel: 561-622-7554, Email: [email protected] May 17-20 Jefferson 500 – Summit Point, WV VRG Drivers School, Royale FF Challenge Series, VeeRG Challenge Series, IMSA RS/Trans Am 2.5 Reunion, VRG Drivers School (May 16-17) with FREE Open Test Day (May 17) Co-Event Chairmen: Cal Trumbo & Jim Karamanis Tel: 304-449-7050 Email: [email protected] June 21-23 5th Annual Vintage Motorsports Festival – Thompson Speedway Motorsports Park, Thompson, CT Oldest Road Course in the USA, Co-Sanctioned with the VSCCA Event Chairman: Paul King, (508) 847-4809, [email protected], July 27-29 New Jersey Historics – NJMP Thunderbolt, Millville, NJ Royale Formula Ford Feature Race, VeeRG Challenge Series Event Chairman: Butch O’Connor, Tel: 973-295-3674, Email: [email protected] September 7-9 VRG at Pitt-Race – Wampum, PA Full Track at PIRC. Royale Formula Ford Feature Race VeeRG Challenge Series Finale Event Chairman: Keith Lawrence, Tel: 412-770-8267, Email: [email protected] October 5-7 VRG at The Glen – Watkins Glen, NY Royale Formula Ford Feature Race Event Chairman: Mike Lawton, Tel: 978-274-5935, Email: [email protected] ü November 23-25 Annual Turkey Bowl XXII – Summit Point, WV Event Chairman: Michael Oritt, Tel: 305-420-4929, Email: [email protected] 1 VINTAGE RACER GROUP NEWSLETTER WATKINS GLEN 2018 IT NEVER GOES AWAY by Bill Stoler Every time I drive under the tunnel and into the infield hear the stories from those of you that were there back at Watkins Glen, it feels surreal. -

Weekly Plan Group B Times Monday Tuesday Wednesday Thursday Friday
Weekly Plan Group b Times Monday Tuesday Wednesday Thursday Friday 8:00-8:15 Community Time Community Time Community Time Community Time Community Time Color Key 8:15-8:35 Math Mini Lesson Math Mini Lesson Math Mini Lesson Math Mini Lesson Math Mini Lesson Whole Group (Zoom) 8:35-9:00 Small Group (Zoom) 9:00-9:20 Math Small Group 1 (Groups 2 and 3 are Math Small Group 1 (Groups 2 and 3 Math Small Group 1 (Groups 2 and 3 Math Small Group 1 (Groups 2 and 3 Math Small Group 1 (Groups 2 and 3 are doing REFLEX and Seesaw Assignment) are doing REFLEX and Seesaw Assignment) are doing REFLEX and Seesaw Assignment) are doing REFLEX and Seesaw Assignment) doing REFLEX and Seesaw Assignment) Pre-Recorded 9:30-9:50 Math Small Group 2 Math Small Group 2 Math Small Group 2 Math Small Group 2 Math Small Group 2 (Groups 2 and 3 are doing REFLEX and Seesaw (Groups 2 and 3 are doing REFLEX and Seesaw (Groups 2 and 3 are doing REFLEX and Seesaw (Groups 2 and 3 are doing REFLEX and Seesaw (Groups 2 and 3 are doing REFLEX and Seesaw Specials (Zoom) Assignment) Assignment) Assignment) Assignment) Assignment) Lunch 10:00-10:30 Math Small Group 3 Math Small Group 3 Math Small Group 3 Math Small Group 3 Math Small Group 3 (Groups 2 and 3 are doing REFLEX and Seesaw (Groups 2 and 3 are doing REFLEX and Seesaw (Groups 2 and 3 are doing REFLEX and Seesaw (Groups 2 and 3 are doing REFLEX and Seesaw (Groups 2 and 3 are doing REFLEX and Seesaw Assignment) Assignment) Assignment) Assignment) Assignment) 11:00-12:00 Lunch Lunch Lunch Lunch Lunch 12:45-1:10 Interactive -

Conjugacy Classes
CONJUGATION IN A GROUP KEITH CONRAD 1. Introduction A reflection across one line in the plane is, geometrically, just like a reflection across every other line. That is, while reflections across two different lines in the plane are not strictly the same, they have the same type of effect. Similarly, two different transpositions in Sn are not the same permutation but have the same type of effect: swap two elements and leave everything else unchanged. The concept that makes the notion of “different, but same type of effect” precise is called conjugacy. In a group G, two elements g and h are called conjugate when h = xgx−1 for some x 2 G. This relation is symmetric, since g = yhy−1 with y = x−1. When h = xgx−1, we say x conjugates g to h. (Warning: when some people say \x conjugates g to h" they might mean h = x−1gx instead of h = xgx−1.) −1 Example 1.1. In S3, what are the conjugates of (12)? We make a table of σ(12)σ for all σ 2 S3. σ (1) (12) (13) (23) (123) (132) σ(12)σ−1 (12) (12) (23) (13) (23) (13) The conjugates of (12) are in the second row: (12), (13), and (23). Notice the redundancy in the table: each conjugate of (12) arises in two ways. We will see in Theorem 5.4 that in Sn all transpositions are conjugate to each other. In AppendixA is a proof that the reflections across each of two lines in the plane are conjugate to each other in the group of all isometries of the plane. -

S.V.R.A Coronado Speed Festival S.V.R.A
S.V.R.A Coronado Speed Festival S.V.R.A PROVISIONAL SCHEDULE Naval Air Station North Island SVRA Sprint Race Series September 18-20, 2015 Race Group 1: Small bore prod. Sports cars and sedans. (SVRA Gr.1) Race Group 2: Formula Ford and Formula B cars. SVRA Gr.2 Race Group 3: World Sports Cars, GT, Prototype and Can-Am Cars 1960-1979 (SVRA Gr. 5+7) Race Group 4: Big Bore Production and Sports Cars through 1972. (SVRA Gr. 6.) Race Group 5: Medium bore Production and Sports cars prior 1972 (SVRA Gr. 3+4) Race Group 6: Series Production Sports Cars and Sedans prior to 1979. (SVRA Gr. 8.) Race Group 7: Wings and Slicks Formula cars. (SVRA Gr.9.) Race Group 8: Historic Stock cars. (SVRA Gr. 10- SC1/2/3.) Race Group 9: Pre war Sports cars and Formula cars Race group 10: Historic Trans Am cars Thursday, September 17 Sunday, September 20 12.00 noon – 5.00 pm Paddock Open for Participant Set-up. 8.00 am - 1.00 pm Registration open 12.00 noon – 5.00 pm Registration open 8.00 am Group 1 warm up 20 min. 12.00 noon -- 5.00 pm Tech 8.20 am Group 2 Friday, September 18 8.40 am Group 3 7.00 am – 5.00 pm Registration open. 9.00 am Group 4 7.00 am – 5 pm Tech Inspection. 9.20 am Group 5 12.00 Mandatory drivers meeting 9.40 am Group 6 1.00 pm Race group 1 Untimed Practice 25 min 10.00 am Break - BMW hot laps 1.25 pm Race group 2 10.15 am Group 7 1.50 pm Race group 3 10.35 am Group 8 2.15 pm Race group 4 10.55 am Group 9 2.40 pm Race group 5 11.15 am Group 10 3.05 pm Race group 6 11.35 am Break – BMW hot laps 3.30 pm Race group 7 3.55 pm Race group 8 11.50 am – 12.50 pm Lunch break 4.20 pm Race group 9 12.10 pm – 12.50 pm Pre Grid Ceremonies w/ H T/A on grid 4.45 pm Race group 10 Saturday, September 19 12.50 pm Group 10 HTA Trophy race 25 min 7.00 am – 4.00pm Registration open. -

Weathertech® International Challenge with Brian Redman July 15 – 18, 2021 Final Schedule (7/12/2021)
WeatherTech® International Challenge with Brian Redman July 15 – 18, 2021 Final Schedule (7/12/2021) Tuesday, July 13, 2021 Friday, July 16, 2021 7:00 AM – 6:00 PM Competitor Registration & Parking 7:00 AM – 4:00 PM Competitor Registration & Parking 7:30 AM – 5:00 PM Technical Inspection (CTECH Scale House) 12 NOON MANDATORY GREEN TEST DRIVERS MEETING (at Victory Lane) QUALIFYING SESSIONS 2 1:00 PM – 5:00 PM Optional GREEN special testing sessions 7:30 AM (see Test Day Schedule) 8:00 AM Group 8 8:25 AM Group 3 Wednesday, July 14, 2021 8:50 AM Group 6 1:00 PM – 7:00 PM Tech Inspection (CTECH Scale House next to Garage) 9:15 AM Group 14 Masters F/1 9:40 AM Group 10 7:00 AM – 6:00 PM Competitor Registration & Parking 10:05 AM Group 9 8:00 AM – 11:40 AM Optional GREEN special testing sessions 10:30 AM Group 2 (See Test Day Schedule) 10:55 AM Group 4 11:20 AM Group 1a 11:40 AM – 12:40 PM Lunch 11:45 AM Group 12 (PreWar/Early PostWar) 12 NOON MANDATORY BLUE TEST DRIVERS MEETING 11:45 AM – 12:55 PM Lunch/Track Touring (at Victory Lane) 12:00 PM MANDATORY DRIVERS MEETING 12:40 PM – 5:45 PM Optional BLUE special testing sessions RA CENTER (see Test Day Schedule) 12:55 PM Group 11 Thursday, July 15, 2021 1:20 PM Group 5 7:00 AM – 5:00 PM Competitor Registration & Parking 1:45 PM Group 7 2:10 PM Group 1b 7:30 AM – 5:00 PM Technical Inspection (CTECH Scale House) QUALIFYING SESSIONS 3 8:00 AM – 10:30 AM Optional BLUE special testing sessions 2:35 PM Group 2 (see Test Day Schedule) 3:00 PM Group 8 10:30 AM Masters E.L. -

Goodyear Racing Staff
2009 Racing Media Guide Table of Contents Goodyear Racing Staff _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ 4 Goodyear Firmly Committed to Racing _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ 6 NASCAR: Goodyear’s Marketing Vehicle _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ 7 Fast Moving and Constantly Changing: Racing and Goodyear _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ 8 From the Track to the Street, Authentic Track-to-Street Innovation _ _ _ _ _ _ _ _ _ _ _ _ _ _ 10 Venue Groupings for Goodyear Eagle and Wrangler Racing Radials _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ 11 Anatomy of a Tire Test _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ 12 Race Tire Sticker Data, NASCAR Tire Cutaway, Passenger Tire Cutaway _ _ _ _ _ _ _ _ _ _ _ _ 15 Goodyear Keeps Drag Racing up to Speed _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ 16 The Big Streak in Pro Stock_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ 18 D2550 Drag Tire: Goodyear Unveils Another Winner _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ 19 Sports Cars: Different Cars, Different Applications, Same Great Results _ _ _ _ _ _ _ _ _ _ _ 20 Short Track: Goodyear’s Long Reach on Short Tracks _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ 22 Dirt Track Racing: Crowd-Pleasing Action on Goodyear Tires_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ 24 Fast Facts -

Victory Lane Magazine
THE VINTAGE AND HISTORIC RACING NEWS MAGAZINE ® Vintage Race Reports. Schedule. Cars for Sale, Auctions and More September 2019: Volume 34, No. 9 89$6.95 USA, $7.95 Canada Road America Pittsburgh Vintage Grand Prix Schenley Park GARDENA, CA GARDENA, PERMIT 40 PERMIT PAID U.S. POSTAGE U.S. PRESORTED STD PRESORTED SOVREN at Pacific Raceways #314 Jim Guthrie, 1966 Shelby GT350; #98 Gary Moore, 1966 Shelby GT350. included a wide variety of cars related to the cannot be raced safely on the street circuit at 2019 PVGP legendary Carroll Shelby. The celebration of Schenley Park. Shelby included high performance track days, Historics a road rally, a poker run, car shows, parade laps PittRace, and a special PVGP Shelby Marque of the Pennsylvania Year vintage race. The July 11-14, 2019 Allegheny Chapter of the BMW Car Club – story and photos by Bill Stoler of America, long-time supporters of the PVGP The 2019 Pittsburgh Vintage Grand Prix also conducted races kicked off the racing July 11-14 at the PittRace during the weekend. Complex, which also served as the site for The Pittsburgh SAAC 44 and the Team Shelby Convention. Vintage Grand Prix The joint meeting of the Shelby American began as a one-day Automobile Club and the Team Shelby vintage racing event #127 Bob Schaefer, 1965 MGB; #4 John Hawkes, East Coast Nationals brought hundreds of on the city streets of 1964 Merlyn Mk 6A. cars to the Beaver County racetrack and Schenley Park in 1983. Over the years, the PVGP Thursday was test and tune day was plagued has expanded to a 10-day by some powerful storms that swept through celebration of the automobile the region but the rest of the weekend was with numerous car shows, road rain-free. -

Barber Motorsports Park – V3 April 3- 6, 2021
Spring Training – Barber Motorsports Park – V3 April 3- 6, 2021 Saturday, April 3 8:00 am - 4:00 pm Registration Open (Porsche Building) 10:00 am USF2000 / Indy Pro 2000 Team Load-In 12:00 pm - 3:00 pm USF2000 / Indy Pro 2000 Driver Photoshoots / Filming (Mandatory) - Set times to be scheduled for each team – 3rd Floor Media Center / Race Control Building 1:00 pm - 5:30 pm Tech Open - USF2000 / Indy Pro 2000 3:15 pm - 4:00 pm USF2000 / Indy Pro 2000 Driver Presentation – Mandatory (Drivers, team representative and coaches welcome) - 2nd Floor Hospitality Room / Race Control Building 3:30 pm - 5:00 pm Tire Scanning - USF2000 / Indy Pro 2000 4:00 pm - 5:00 pm Track Walk (No Vehicles or Bicycles, Walking Only!) 5:15 pm - 5:30 pm USF2000 / Indy Pro 2000 - RTI TV 2021 Overview (Mandatory) - 3rd Floor Media Center 5:30 pm - 5:45 pm SimMetric Driver Performance Lab Presentation (Mandatory) - 3rd Floor Media Center 6:00 pm - 7:00 pm Welcome Reception - 2nd Floor Hospitality Room / Race Control Building Sunday, April 4 9:00 am - 4:00 pm Registration Open (Porsche Building) 9:00 am - 10:00 am Tech Open - USF2000 / Indy Pro 2000 9:00 am - 12:00 pm USF2000 & Indy Pro 2000 Driver weigh-in (Mandatory Sunday or Monday) - fully suited with helmet and hans 10:00 am - 10:30 am Easter Service hosted by Chuck Lessick / IndyCar Ministry (2nd Floor Race Control Bldg) 11:00 am - 12:00 pm Tech Open - USF2000 / Indy Pro 2000 12:00 pm Engine Start / No engines running before noon 12:00 pm - 12:45 pm Indy Pro 2000 Group 1 – Cooper Tires Presentation (Mandatory) -
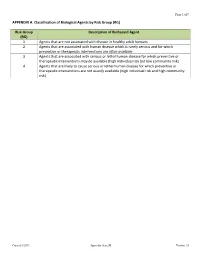
APPENDIX A. Classification of Biological Agents by Risk Group (RG)
Page 1 of 9 APPENDIX A. Classification of Biological Agents by Risk Group (RG) Risk Group Description of Biohazard Agent (RG) 1 Agents that are not associated with disease in healthy adult humans 2 Agents that are associated with human disease which is rarely serious and for which preventive or therapeutic interventions are often available 3 Agents that are associated with serious or lethal human disease for which preventive or therapeutic interventions may be available (high individual risk but low community risk) 4 Agents that are likely to cause serious or lethal human disease for which preventive or therapeutic interventions are not usually available (high individual risk and high community risk) Created 3/2013 Appendix A and B Version 1.0 Page 2 of 9 APPENDIX B. Risk Group (RG) Agents If your project involves the use of bacteria, fungi, virus, animal etiologic virus not listed below, please contact the Biosafety Officer at 323-563-5913 or write to [email protected] before you start the research. Any research project involving the use of biological agents in the RG3 or RG4 below cannot be conducted at CDU. (All Appendix citations in this section are from NIH Guidelines for Recombinant DNA at http://oba.od.nih.gov/oba/rac/Guidelines/NIH_Guidelines_prnnew.pdf) RISK GROUP 1 (RG1) Agents Agents that are not associated with disease in healthy adult humans. Some examples are, AsporogenicBacillus subtilisor Bacillus licheniformis (see Appendix C-IV-A, Bacillus subtilisor Bacillus licheniformisHost-Vector Systems, Exceptions) Adeno- associated virus (AAV) types 1 through 4 Recombinant AAV constructs, in which the transgene does not encode either a potentially tumorigenic gene product or a toxin molecule and are produced in the absence of a helper virus. -

Weathertech® International Challenge with Brian Redman July 15 – 18, 2021 Provisional Schedule (2/2/2021)
WeatherTech® International Challenge with Brian Redman July 15 – 18, 2021 Provisional Schedule (2/2/2021) Tuesday, July 13, 2021 Friday, July 16, 2021 8:00 AM – 6:00 PM Competitor Registration & Parking 7:00 AM – 4:00 PM Competitor Registration & Parking 7:30 AM – 5:00 PM Technical Inspection (CTECH Scale House) 12 NOON MANDATORY GREEN TEST DRIVERS MEETING (at Victory Lane) QUALIFYING SESSIONS 2 1:00 PM – 5:00 PM Optional GREEN special testing sessions 8:00 AM Group 4 (see Test Day Schedule) 8:25 AM Group 1B 8:50 AM Group 6 Wednesday, July 14, 2021 9:15 AM Group 10 1:00 PM – 7:00 PM Tech Inspection (CTECH Scale House next to Garage) 9:40 AM Group 9/MEL (Q session 1) 10:05 AM Group 5 8:00 AM – 6:00 PM Competitor Registration & Parking 10:30 AM Group 1A 8:00 AM – 11:40 AM Optional GREEN special testing sessions 10:55 AM Group 3 (See Test Day Schedule) 11:20 AM Group 8 11:40 AM – 12:40 PM Lunch 11:45 AM – 12:45 PM Lunch/Track Touring 12 NOON MANDATORY BLUE TEST DRIVERS MEETING 12:45 PM Group 11 (at Victory Lane) 1:10 PM Group 2 12:40 PM – 5:45 PM Optional BLUE special testing sessions 1:35 PM Groups 7A/B/C/D (see Test Day Schedule) 2:00 PM Groups 9/MEL Thursday, July 15, 2021 QUALIFYING SESSIONS 3 7:00 AM – 5:00 PM Competitor Registration & Parking 2:25 PM Group 2 2:50 PM Group 4 7:30 AM – 5:00 PM Technical Inspection (CTECH Scale House) 3:15 PM Group 6 3:40 PM Group 3 8:00 AM – 10:30 AM Optional BLUE special testing sessions 4:05 PM Group 10 (see Test Day Schedule) 4:30 PM Group 8 10:30 AM Masters E.L.