Mycotic Keratitis—A Global Threat from the Filamentous Fungi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
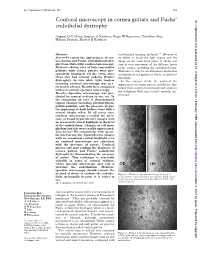
Confocal Microscopy in Cornea Guttata and Fuchs' Endothelial Dystrophy
Br J Ophthalmol 1999;83:185–189 185 Confocal microscopy in cornea guttata and Fuchs’ Br J Ophthalmol: first published as 10.1136/bjo.83.2.185 on 1 February 1999. Downloaded from endothelial dystrophy Auguste G-Y Chiou, Stephen C Kaufman, Roger W Beuerman, Toshihiko Ohta, Hisham Soliman, Herbert E Kaufman Abstract conventional imaging methods.3–13 Because of Aims—To report the appearances of cor- its ability to focus the light source and the nea guttata and Fuchs’ endothelial dystro- image on the same focal plane, it allows real phy from white light confocal microscopy. time in vivo assessment of the diVerent layers Methods—Seven eyes of four consecutive of the cornea, including the endothelial layer. patients with cornea guttata were pro- Therefore, it may be an alternative method in spectively examined. Of the seven eyes, evaluating cornea guttata or Fuchs’ endothelial three also had corneal oedema (Fuchs’ dystrophy. dystrophy). In vivo white light tandem In the current study, we analysed the scanning confocal microscopy was per- appearances of cornea guttata and Fuchs’ dys- formed in all eyes. Results were compared trophy from confocal microscopy and compare with non-contact specular microscopy. the technique with non-contact specular mi- Results—Specular microscopy was pre- croscopy. cluded by corneal oedema in one eye. In the remaining six eyes, it demonstrated typical changes including pleomorphism, polymegathism, and the presence of gut- tae appearing as dark bodies, some with a central bright reflex. In all seven eyes, confocal microscopy revealed the pres- ence of round hyporeflective images with an occasional central highlight at the level of the endothelium. -

Differentiate Red Eye Disorders
Introduction DIFFERENTIATE RED EYE DISORDERS • Needs immediate treatment • Needs treatment within a few days • Does not require treatment Introduction SUBJECTIVE EYE COMPLAINTS • Decreased vision • Pain • Redness Characterize the complaint through history and exam. Introduction TYPES OF RED EYE DISORDERS • Mechanical trauma • Chemical trauma • Inflammation/infection Introduction ETIOLOGIES OF RED EYE 1. Chemical injury 2. Angle-closure glaucoma 3. Ocular foreign body 4. Corneal abrasion 5. Uveitis 6. Conjunctivitis 7. Ocular surface disease 8. Subconjunctival hemorrhage Evaluation RED EYE: POSSIBLE CAUSES • Trauma • Chemicals • Infection • Allergy • Systemic conditions Evaluation RED EYE: CAUSE AND EFFECT Symptom Cause Itching Allergy Burning Lid disorders, dry eye Foreign body sensation Foreign body, corneal abrasion Localized lid tenderness Hordeolum, chalazion Evaluation RED EYE: CAUSE AND EFFECT (Continued) Symptom Cause Deep, intense pain Corneal abrasions, scleritis, iritis, acute glaucoma, sinusitis, etc. Photophobia Corneal abrasions, iritis, acute glaucoma Halo vision Corneal edema (acute glaucoma, uveitis) Evaluation Equipment needed to evaluate red eye Evaluation Refer red eye with vision loss to ophthalmologist for evaluation Evaluation RED EYE DISORDERS: AN ANATOMIC APPROACH • Face • Adnexa – Orbital area – Lids – Ocular movements • Globe – Conjunctiva, sclera – Anterior chamber (using slit lamp if possible) – Intraocular pressure Disorders of the Ocular Adnexa Disorders of the Ocular Adnexa Hordeolum Disorders of the Ocular -

Curvularia Keratitis*
09 Wilhelmus Final 11/9/01 11:17 AM Page 111 CURVULARIA KERATITIS* BY Kirk R. Wilhelmus, MD, MPH, AND Dan B. Jones, MD ABSTRACT Purpose: To determine the risk factors and clinical signs of Curvularia keratitis and to evaluate the management and out- come of this corneal phæohyphomycosis. Methods: We reviewed clinical and laboratory records from 1970 to 1999 to identify patients treated at our institution for culture-proven Curvularia keratitis. Descriptive statistics and regression models were used to identify variables associ- ated with the length of antifungal therapy and with visual outcome. In vitro susceptibilities were compared to the clini- cal results obtained with topical natamycin. Results: During the 30-year period, our laboratory isolated and identified Curvularia from 43 patients with keratitis, of whom 32 individuals were treated and followed up at our institute and whose data were analyzed. Trauma, usually with plants or dirt, was the risk factor in one half; and 69% occurred during the hot, humid summer months along the US Gulf Coast. Presenting signs varied from superficial, feathery infiltrates of the central cornea to suppurative ulceration of the peripheral cornea. A hypopyon was unusual, occurring in only 4 (12%) of the eyes but indicated a significantly (P = .01) increased risk of subsequent complications. The sensitivity of stained smears of corneal scrapings was 78%. Curvularia could be detected by a panfungal polymerase chain reaction. Fungi were detected on blood or chocolate agar at or before the time that growth occurred on Sabouraud agar or in brain-heart infusion in 83% of cases, although colonies appeared only on the fungal media from the remaining 4 sets of specimens. -

Original Article
Clinical and Experimental Ophthalmology 2007; 35: 124–130 doi:10.1111/j.1442-9071.2006.01405.x Original Article Fungal keratitis in Melbourne Prashant Bhartiya FRCS,1,2 Mark Daniell FRANZCO,1,2 Marios Constantinou BScHons BOrth,1,2 FM Amirul Islam PhD1,2 and Hugh R Taylor AC FRANZCO1,2 1Centre for Eye Research Australia, University of Melbourne, and 2Corneal Clinic, Royal Victorian Eye and Ear Hospital, Melbourne, Victoria, Australia ABSTRACT INTRODUCTION Background: Description of the clinical and microbiolog- Fungal keratitis is a potentially blinding ocular disease. The ical spectrum of fungal keratitis at a tertiary eye care hos- incidence of fungal keratitis varies widely throughout the pital in Melbourne, Australia. world. A report from India showed that nearly 50% of all corneal ulcers were caused by fungi.1 This high prevalence Methods: Retrospective review of all patients with keratitis of fungal pathogens in south India is significantly greater with positive fungal cultures from corneal or associated than that found in similar studies in Nepal (17%),2 samples presenting to the Royal Victorian Eye and Ear Hos- Bangladesh (36%)3 and south Florida (35%).4 Several large pital, Melbourne, Australia from July 1996 to May 2004. studies on fungal keratitis have been published from North 4–12 Demographic data, predisposing factors, features on pre- and South America, Africa and the Indian subcontinent. However, there is a paucity of data on the spectrum of fungal sentation, management, outcomes and microbiological data keratitis in patients from Australia. This study reviewed a were collected and analysed. series of patients with keratitis who had fungal growth on Results: The study included 56 eyes of 56 patients. -

Onchocerciasis
11 ONCHOCERCIASIS ADRIAN HOPKINS AND BOAKYE A. BOATIN 11.1 INTRODUCTION the infection is actually much reduced and elimination of transmission in some areas has been achieved. Differences Onchocerciasis (or river blindness) is a parasitic disease in the vectors in different regions of Africa, and differences in cause by the filarial worm, Onchocerca volvulus. Man is the the parasite between its savannah and forest forms led to only known animal reservoir. The vector is a small black fly different presentations of the disease in different areas. of the Simulium species. The black fly breeds in well- It is probable that the disease in the Americas was brought oxygenated water and is therefore mostly associated with across from Africa by infected people during the slave trade rivers where there is fast-flowing water, broken up by catar- and found different Simulium flies, but ones still able to acts or vegetation. All populations are exposed if they live transmit the disease (3). Around 500,000 people were at risk near the breeding sites and the clinical signs of the disease in the Americas in 13 different foci, although the disease has are related to the amount of exposure and the length of time recently been eliminated from some of these foci, and there is the population is exposed. In areas of high prevalence first an ambitious target of eliminating the transmission of the signs are in the skin, with chronic itching leading to infection disease in the Americas by 2012. and chronic skin changes. Blindness begins slowly with Host factors may also play a major role in the severe skin increasingly impaired vision often leading to total loss of form of the disease called Sowda, which is found mostly in vision in young adults, in their early thirties, when they northern Sudan and in Yemen. -

Review the Global Incidence and Diagnosis of Fungal Keratitis
Review The global incidence and diagnosis of fungal keratitis Lottie Brown, Astrid K Leck, Michael Gichangi, Matthew J Burton, David W Denning Fungal keratitis is a severe corneal infection that often results in blindness and eye loss. The disease is most prevalent Lancet Infect Dis 2020 in tropical and subtropical climates, and infected individuals are frequently young agricultural workers of low Published Online socioeconomic status. Early diagnosis and treatment can preserve vision. Here, we discuss the fungal keratitis October 22, 2020 diagnostic literature and estimate the global burden through a complete systematic literature review from January, 1946 https://doi.org/10.1016/ S1473-3099(20)30448-5 to July, 2019. An adapted GRADE score was used to evaluate incidence papers—116 studies provided the incidence of University of Manchester, fungal keratitis as a proportion of microbial keratitis and 18 provided the incidence in a defined population. We Manchester, UK (L Brown MSc, calculated a minimum annual incidence estimate of 1 051 787 cases (736 251–1 367 323), with the highest rates in Asia Prof D W Denning FRCP); and Africa. If all culture-negative cases are assumed to be fungal, the annual incidence would be 1 480 916 cases International Centre for Eye (1 036 641–1 925 191). In three case series, 8–11% of patients had to have the eye removed, which represents an annual Health, London School of Hygiene & Tropical Medicine, loss of 84 143–115 697 eyes. As fungal keratitis probably affects over a million people annually, an inexpensive, simple London, UK (A K Leck PhD, diagnostic method and affordable treatment are needed in every country. -

Topical Corticosteroids and Fungal Keratitis: a Review of the Literature and Case Series
Journal of Clinical Medicine Review Topical Corticosteroids and Fungal Keratitis: A Review of the Literature and Case Series Karl Anders Knutsson 1,*, Alfonso Iovieno 2,3, Stanislav Matuska 1, Luigi Fontana 2 and Paolo Rama 1 1 Cornea and Ocular Surface Unit, San Raffaele Scientific Institute, 20132 Milan, Italy; [email protected] (S.M.); [email protected] (P.R.) 2 Arcispedale Santa Maria Nuova—IRCCS, 42123 Reggio Emilia, Italy; [email protected] (A.I.); [email protected] (L.F.) 3 Department of Ophthalmology, University of British Columbia, Vancouver, BC V6T 1Z, Canada * Correspondence: [email protected] or [email protected]; Tel./Fax: +39-022-6432-648 Abstract: The management of fungal keratitis is complex since signs and symptoms are subtle and ocular inflammation is minimal in the preliminary stages of infection. Initial misdiagnosis of the condition and consequent management of inflammation with corticosteroids is a frequent occurrence. Topical steroid use is considered to be a principal factor for development of fungal keratitis. In this review, we assess the studies that have reported outcomes of fungal keratitis in patients receiving steroids prior to diagnosis. We also assess the possible rebound effect present when steroids are abruptly discontinued and the clinical characteristics of three patients in this particular clinical scenario. Previous reports and the three clinical descriptions presented suggest that in fungal keratitis, discontinuing topical steroids can induce worsening of clinical signs. In these cases, we recommend to slowly taper steroids and continue or commence appropriate antifungal therapy. Citation: Knutsson, K.A.; Iovieno, A.; Keywords: fungal keratitis; topical corticosteroids; topical steroids; rebound effect Matuska, S.; Fontana, L.; Rama, P. -

Fusarium Keratitis and Corneal Collagen Cross
FUSARIUM KERATITIS AND SURGERY REFRACTIVE CORNEAL COLLAGEN CROSS-LINKING BY MINAS CORONEO, AO, BSC(MED), MBBS, MSC SYD, MD, MS, UNSW, FRACS, FRANZCO; RAJESH FOGLA, DNB, FRCS(EDIN), MMED(OPHTH); WILLIAM B. TRATTLER, MD; ASHIYANA NARIANI, MD, MPH; COMPLEX CASE MANAGEMENT COMPLEX GARGI KHARE VORA, MD; AND ALAN N. CARLSON, MD CASE PRESENTATION A 42-year-old white man is referred to the Duke University Eye Center Cornea Service for a central corneal ulcer with a hypopyon in his right eye. The patient sustained the ocular injury while mowing the lawn, with debris getting into the eye while he was wearing contact lenses. He was diagnosed with culture-positive Fusarium species by the referring ophthalmologist and was treat- ed with oral voriconazole 200 mg twice daily and frequent topical natamycin 5% and voriconazole 10 mg/mL. The patient under- went epithelium-off corneal collagen cross-linking (CXL) approxi- Figure 1. Initial evaluation of the eye with a Fusarium corneal mately 4 weeks after diagnosis of the ulcer and was treated with infiltrate and hypopyon. a loteprednol steroid taper after the procedure. His condition subsequently progressed, with increasing eye pain, a nonhealing epithelial defect, and a worsening corneal infiltrate. Upon evaluation, the patient has a large corneal infiltrate with necrotic stroma, which is approaching the limbus, and a hypopyon (Figure 1). His UCVA measures 20/70-1. B-scan ultrasound of the right eye shows no evidence of posterior segment involvement. Reculturing of the corneal infiltrate is negative for bacteria, fungus, and Acanthamoeba. Confocal microscopy reveals no evidence of hyphae or cysts. -

Immune Defense at the Ocular Surface
Eye (2003) 17, 949–956 & 2003 Nature Publishing Group All rights reserved 0950-222X/03 $25.00 www.nature.com/eye Immune defense at EK Akpek and JD Gottsch CAMBRIDGE OPHTHALMOLOGICAL SYMPOSIUM the ocular surface Abstract vertebrates. Improved visual acuity would have increased the fitness of these animals and would The ocular surface is constantly exposed to a have outweighed the disadvantage of having wide array of microorganisms. The ability of local immune cells and blood vessels at a the outer ocular system to recognize pathogens distance where a time delay in addressing a as foreign and eliminate them is critical to central corneal infection could lead to blindness. retain corneal transparency, hence The first vertebrates were jawless fish that preservation of sight. Therefore, a were believed to have evolved some 470 million combination of mechanical, anatomical, and years ago.1 These creatures had frontal eyes and immunological defense mechanisms has inhabited the shorelines of ancient oceans. With evolved to protect the outer eye. These host better vision, these creatures were likely more defense mechanisms are classified as either a active and predatory. This advantage along with native, nonspecific defense or a specifically the later development of jaws enabled bony fish acquired immunological defense requiring to flourish and establish other habitats. One previous exposure to an antigen and the such habitat was shallow waters where lunged development of specific immunity. Sight- fish made the transition to land several hundred threatening immunopathology with thousand years later.2 To become established in autologous cell damage also can take place this terrestrial environment, the new vertebrates after these reactions. -

Herpetic Corneal Infections
FocalPoints Clinical Modules for Ophthalmologists VOLUME XXVI, NUMBER 8 SEPTEMBER 2008 (MODULE 2 OF 3) Herpetic Corneal Infections Sonal S. Tuli, MD Reviewers and Contributing Editors Consultants George A. Stern, MD, Editor for Cornea & External Disease James Chodosh, MD, MPH Kristin M. Hammersmith, MD, Basic and Clinical Science Course Faculty, Section 8 Kirk R. Wilhelmus, MD, PhD Christie Morse, MD, Practicing Ophthalmologists Advisory Committee for Education Focal Points Editorial Review Board George A. Stern, MD, Missoula, MT Claiming CME Credit Editor in Chief, Cornea & External Disease Thomas L. Beardsley, MD, Asheville, NC Academy members: To claim Focal Points CME cred- Cataract its, visit the Academy web site and access CME Central (http://www.aao.org/education/cme) to view and print William S. Clifford, MD, Garden City, KS Glaucoma Surgery; Liaison for Practicing Ophthalmologists Advisory your Academy transcript and report CME credit you Committee for Education have earned. You can claim up to two AMA PRA Cate- gory 1 Credits™ per module. This will give you a maxi- Bradley S. Foster, MD, Springfield, MA Retina & Vitreous mum of 24 credits for the 2008 subscription year. CME credit may be claimed for up to three (3) years from Anil D. Patel, MD, Oklahoma City, OK date of issue. Non-Academy members: For assistance Neuro-Ophthalmology please send an e-mail to [email protected] or a Eric P. Purdy, MD, Fort Wayne, IN fax to (415) 561-8575. Oculoplastic, Lacrimal, & Orbital Surgery Steven I. Rosenfeld, MD, FACS, Delray Beach, FL Refractive Surgery, Optics & Refraction C. Gail Summers, MD, Minneapolis, MN Focal Points (ISSN 0891-8260) is published quarterly by the American Academy of Ophthalmology at 655 Beach St., San Francisco, CA 94109-1336. -

Treatment of Interface Keratitis with Oral Corticosteroids
Treatment of interface keratitis with oral corticosteroids Scott M. MacRae, MD, Larry F. Rich, MD, Damien C. Macaluso, MD ABSTRACT Purpose: To describe the results of treating interface keratitis using a combination of intensive topical and oral corticosteroids. Setting: Casey Eye Institute, Portland, Oregon, USA. Methods: Thirteen eyes treated for grade 2 to 3 interface keratitis using an oral cortico- steroid (prednisone 60 to 80 mg) as well as an hourly topical corticosteroid were retrospectively reviewed. The best corrected visual acuity (BCVA) was used as an objective guide of whether to treat with intense topical and oral corticosteroids, flap irrigation, or both. Predisposing factors such as intraoperative epithelial defects or a history of severe allergies or atopy were also looked for. Results: All 13 eyes responded favorably to the combination of intensive topical and oral corticosteroids and had a BCVA of 20/20 after the keratitis resolved. In 6 eyes (46%), the patients had a history of severe seasonal allergies. One day postoperatively, 3 eyes (23%) had an epithelial defect and 2 eyes (15%), lint particles or debris embedded in the interface. With oral corticosteroid use, 3 patients (23%) noted mild stomach irritation and 2 (15%) noted nervousness. All 5 side effects resolved without sequelae. No patient developed a serious side effect. Conclusion: A short, intense course of an oral corticosteroid was an effective treatment in patients with grade 2 or higher interface keratitis when combined with a topical corti- costeroid administered hourly. The BCVA is a helpful objective measure of the severity of interface keratitis and can be used to guide the clinician in the therapeutic strategy. -

Infectious Keratitis: Short Answers
Q Infectious keratitis: Short answers What is the #1 bacterium in CL-related K ulcer? A Infectious keratitis: Short answers What is the #1 bacterium in CL-related K ulcer? Pseudomonas Infectious keratitis: Short answers Pseudomonas corneal ulcer associated with CL wear Q Infectious keratitis: Short answers What is the #1 bacterium in CL-related K ulcer? Pseudomonas What is the #1 risk factor for Acanthamoeba keratitis? A Infectious keratitis: Short answers What is the #1 bacterium in CL-related K ulcer? Pseudomonas What is the #1 risk factor for Acanthamoeba keratitis? CL wear Infectious keratitis: Short answers Acanthamoeba keratitis associated with CL wear Q Infectious keratitis: Short answers What is the #1 bacterium in CL-related K ulcer? Pseudomonas What is the #1 risk factor for Acanthamoeba keratitis? CL wear What are the three main culprits in fungal keratitis? What is the topical antifungal of choice for each? Fusarium:fungus 1 Topical…natamycin Aspergillisfungus 2 and Candida:fungus 3 A Infectious keratitis: Short answers What is the #1 bacterium in CL-related K ulcer? Pseudomonas What is the #1 risk factor for Acanthamoeba keratitis? CL wear What are the three main culprits in fungal keratitis? What is the topical antifungal of choice for each? Candida: Topical…natamycin Aspergillis and Fusarium: Q Infectious keratitis: Short answers What is the #1 bacterium in CL-related K ulcer? Pseudomonas What is the #1 risk factor for Acanthamoeba keratitis? CL wear What are the three main culprits in fungal keratitis?