Connecting the Dots: a Food Web of the Lower Ogeechee River
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ogeechee River
I ) f'"I --- , ',, ', ' • ''i' • ;- 1, '\::'.e...,. " .; IL. r final wild and s~;ni;ri~~f'1tu~; MAY, 1984 OGEECHEE RIVER GEORGIA L_ - UNITED STATES DEPARI'MENT OF 'IHE INTERIOR/NATIONAL PARK SERVICE As the Nation's principal conservation a gency, the Department of the Interior has responsibility tor most of our nationally owned public lands and natural resources. This includes fostering the wisest use of our land and water resources, protecting our fish and wildlife, preserving the environ mental and cultural values of our national parks and historical places, and providing for the enjoyment of life through out door recreation. The Department assesses our energy and min eral resources and works to assure that their development is • in the best interests of all our people. The Department also has a major responsibility for American Indian reservation communities and for people who live in island territories un der U. S. administration. f Pf /p- I. SUMMARY OF FINDIN:jS / 1 II. CONDUCT OF 'ffiE STUDY / 5 Backgrouoo and Purpose of Study I 5 Study Approach I 5 Public Involvement I 6 III. EVALUATICN / 8 Eligibility I 8 Classification I 8 Suitability I 11 IV. THE RIVER ENVIOC>NMENT / 17 I.ocation and Access / 17 Population I 17 Landownership and Use I 17 Natural Resources / 22 Recreation Resources I 32 Cultural Resources I 35 V. A GUIDE 'IO RIVER PIDTECTICN ALTERNATIVES / 37 VI. LIST OF STUDY PARI'ICIPANI'S AND CXNSULTANI'S / 52 VII. APPENDIX / 54 IWJSTRATIONS/rABLES I.ocation Map I 3 River Classification / 9 Ogeechee River Study Region County Populations / 18 General Land Uses / 19 Typical Ogeechee River Sections Lower Piedmont Segment I 23 Upper Coastal Plain Segment I 24 Lower Coastal Plain / 25 Coastal Marsh I 26 Hydrology I 29 Significant Features I 33 Line-of-Sight Fran the River / 42 I. -

The Georgia Coast Saltwater Paddle Trail
2010 The Georgia Coast Saltwater Paddle Trail This project was funded in part by the Coastal Management Program of the Georgia Department of Natural Resources, and the U.S. Department of Commerce, Office of Ocean and Coastal Resource Management (OCRM), National Oceanic and Atmospheric Administration (NOAA) grant award #NA09NOS4190171, as well as the National Park Service Rivers, Trails & Conservation Assistance Program. The statements, findings, conclusions, and recommendations are those of the authors and do not necessarily reflect the views of OCRM or NOAA. September 30, 2010 0 CONTENTS ACKNOWLEDGEMENTS ......................................................................................................................................... 2 Coastal Georgia Regional Development Center Project Team .......................................................... 3 Planning and Government Services Staff ................................................................................................... 3 Geographic Information Systems Staff ....................................................................................................... 3 Economic Development Staff .......................................................................................................................... 3 Administrative Services Staff .......................................................................................................................... 3 Introduction ............................................................................................................................................................... -

Biological Assessment of the Patapsco River Tributary Watersheds, Howard County, Maryland
Biological Assessment of the Patapsco River Tributary Watersheds, Howard County, Maryland Spring 2003 Index Period and Summary of Round One County- Wide Assessment Patuxtent River April, 2005 Final Report UT to Patuxtent River Biological Assessment of the Patapsco River Tributary Watersheds, Howard County, Maryland Spring 2003 Index Period and Summary of Round One County-wide Assessment Prepared for: Howard County, Maryland Department of Public Works Stormwater Management Division 6751 Columbia Gateway Dr., Ste. 514 Columbia, MD 21046-3143 Prepared by: Tetra Tech, Inc. 400 Red Brook Blvd., Ste. 200 Owings Mills, MD 21117 Acknowledgement The principal authors of this report are Kristen L. Pavlik and James B. Stribling, both of Tetra Tech. They were also assisted by Erik W. Leppo. This document reports results from three of the six subwatersheds sampled during the Spring Index Period of the third year of biomonitoring by the Howard County Stormwater Management Division. Fieldwork was conducted by Tetra Tech staff including Kristen Pavlik, Colin Hill, David Bressler, Jennifer Pitt, and Amanda Richardson. All laboratory sample processing was conducted by Carolina Gallardo, Shabaan Fundi, Curt Kleinsorg, Chad Bogues, Joey Rizzo, Elizabeth Yarborough, Jessica Garrish, Chris Hines, and Sara Waddell. Taxonomic identification was completed by Dr. R. Deedee Kathman and Todd Askegaard; Aquatic Resources Center (ARC). Hunt Loftin, Linda Shook, and Brenda Decker (Tetra Tech) assisted with budget tracking and clerical support. This work was completed under the Howard County Purchase Order L 5305 to Tetra Tech, Inc. The enthusiasm and interest of the staff in the Stormwater Management Division, including Howard Saltzman and Angela Morales is acknowledged and appreciated. -
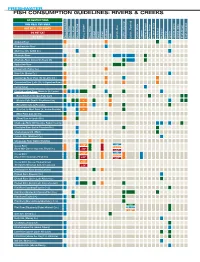
Fish Consumption Guidelines: Rivers & Creeks
FRESHWATER FISH CONSUMPTION GUIDELINES: RIVERS & CREEKS NO RESTRICTIONS ONE MEAL PER WEEK ONE MEAL PER MONTH DO NOT EAT NO DATA Bass, LargemouthBass, Other Bass, Shoal Bass, Spotted Bass, Striped Bass, White Bass, Bluegill Bowfin Buffalo Bullhead Carp Catfish, Blue Catfish, Channel Catfish,Flathead Catfish, White Crappie StripedMullet, Perch, Yellow Chain Pickerel, Redbreast Redhorse Redear Sucker Green Sunfish, Sunfish, Other Brown Trout, Rainbow Trout, Alapaha River Alapahoochee River Allatoona Crk. (Cobb Co.) Altamaha River Altamaha River (below US Route 25) Apalachee River Beaver Crk. (Taylor Co.) Brier Crk. (Burke Co.) Canoochee River (Hwy 192 to Lotts Crk.) Canoochee River (Lotts Crk. to Ogeechee River) Casey Canal Chattahoochee River (Helen to Lk. Lanier) (Buford Dam to Morgan Falls Dam) (Morgan Falls Dam to Peachtree Crk.) * (Peachtree Crk. to Pea Crk.) * (Pea Crk. to West Point Lk., below Franklin) * (West Point dam to I-85) (Oliver Dam to Upatoi Crk.) Chattooga River (NE Georgia, Rabun County) Chestatee River (below Tesnatee Riv.) Chickamauga Crk. (West) Cohulla Crk. (Whitfield Co.) Conasauga River (below Stateline) <18" Coosa River <20" 18 –32" (River Mile Zero to Hwy 100, Floyd Co.) ≥20" >32" <18" Coosa River <20" 18 –32" (Hwy 100 to Stateline, Floyd Co.) ≥20" >32" Coosa River (Coosa, Etowah below <20" Thompson-Weinman dam, Oostanaula) ≥20" Coosawattee River (below Carters) Etowah River (Dawson Co.) Etowah River (above Lake Allatoona) Etowah River (below Lake Allatoona dam) Flint River (Spalding/Fayette Cos.) Flint River (Meriwether/Upson/Pike Cos.) Flint River (Taylor Co.) Flint River (Macon/Dooly/Worth/Lee Cos.) <16" Flint River (Dougherty/Baker Mitchell Cos.) 16–30" >30" Gum Crk. -

Stream-Temperature Charcteristics in Georgia
STREAM-TEMPERATURE CHARACTERISTICS IN GEORGIA U.S. GEOLOGICAL SURVEY Prepared in cooperation with the GEORGIA DEPARTMENT OF NATURAL RESOURCES ENVIRONMENTAL PROTECTION DIVISION Water-Resources Investigations Report 96-4203 STREAM-TEMPERATURE CHARACTERISTICS IN GEORGIA By T.R. Dyar and S.J. Alhadeff ______________________________________________________________________________ U.S. GEOLOGICAL SURVEY Water-Resources Investigations Report 96-4203 Prepared in cooperation with GEORGIA DEPARTMENT OF NATURAL RESOURCES ENVIRONMENTAL PROTECTION DIVISION Atlanta, Georgia 1997 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Charles G. Groat, Director For additional information write to: Copies of this report can be purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Branch of Information Services 3039 Amwiler Road, Suite 130 Denver Federal Center Peachtree Business Center Box 25286 Atlanta, GA 30360-2824 Denver, CO 80225-0286 CONTENTS Page Abstract . 1 Introduction . 1 Purpose and scope . 2 Previous investigations. 2 Station-identification system . 3 Stream-temperature data . 3 Long-term stream-temperature characteristics. 6 Natural stream-temperature characteristics . 7 Regression analysis . 7 Harmonic mean coefficient . 7 Amplitude coefficient. 10 Phase coefficient . 13 Statewide harmonic equation . 13 Examples of estimating natural stream-temperature characteristics . 15 Panther Creek . 15 West Armuchee Creek . 15 Alcovy River . 18 Altamaha River . 18 Summary of stream-temperature characteristics by river basin . 19 Savannah River basin . 19 Ogeechee River basin. 25 Altamaha River basin. 25 Satilla-St Marys River basins. 26 Suwannee-Ochlockonee River basins . 27 Chattahoochee River basin. 27 Flint River basin. 28 Coosa River basin. 29 Tennessee River basin . 31 Selected references. 31 Tabular data . 33 Graphs showing harmonic stream-temperature curves of observed data and statewide harmonic equation for selected stations, figures 14-211 . -

Invertebrate Species List E Xcluding Papilionoidea (Butterflies )
Invertebrate Species List E xcluding Papilionoidea (Butterflies ) Higher Classification1 Kingdom: Animalia Phyllum (P:), Class (C:), Order (O:) and Family (F:)3 Taxa Identified1 English Name2 P: Annelida Segmented Worms C: Oligochaeta Oligochaeta sp. Night Crawlers P: Arthropoda Arthropods C: Arachnida Arachnids O: Araneae Spiders F: Agelenidae Agelenidae sp. Funnel Weavers F: Anyphaenidae Anyphaenidae sp. Ghost Spiders F: Araneidae Araneidae sp. Orb Weavers F: Clubionidae Clubionidae sp. Sac Spiders F: Ctenidae Ctenidae sp. Wandering Spiders F: Dictynidae Dictynidae sp. Meshweavers F: Dipluridae Dipluridae sp. Funnelweb Spiders F: Gnaphosidae Gnaphosidae sp. Stealthy Ground Spiders F: Linyphiidae Linyphiidae sp. Sheetweb Weavers F: Lycosidae Lycosidae sp. Wold Spiders F: Pholcidae Pholcidae sp. Daddy Long-legs Spiders F: Salticidae Salticidae sp. Jumping Spiders F: Tetragnathidae Tetragnathidae sp. Long-jawed Orb Weavers F: Theridiidae Theridiidae sp. Comb-footed Spiders F: Thomisidae Thomisidae sp. Crab Spiders O: Opiliones Opiliones spp. Daddy Longlegs & Harvestmen O: Pseudoscorpiones Pseudoscorpiones sp. Pseudoscorpions iC: Acari Acari sp. Mites supO: Parasitiformes Parasitiformes sp. Ticks C: Chilopoda Chilopoda sp. Centipedes C: Diplopoda Diplopoda sp. Millipedes O: Polydesmida F: Polydesmidae Polydesmus python C: Entognatha Entognathids O: Collembola Springtails F: Poduridae Poduridae sp. Water Springtails F: Sminthuridae Sminthuridae sp. Globular Springtails subO: Entomobryomorpha Entomobryomorpha sp. F: Entomobryidae Entomobryidae sp. Slender Springtails F: Isotomidae Isotomidae sp. Smooth Springtails O: Diplura Diplura sp. O: Protura Protura sp. Proturans Page 1 of 7 Cloudbridge Nature Reserve, Costa Rica Last Updated: August 28, 2018 Invertebrate Species List E xcluding Papilionoidea (Butterflies ) Phyllum (P:), Class (C:), Order (O:) and Family (F:)3 Taxa Identified1 English Name2 P: Arthropoda (cont’d) Arthropods C: Insecta Insects O: Blattodea Cockroaches F: Blattidae Blattidae sp. -

Greater Savannah
80 405 Airways Ave S Coastal Hwy 26 104 21 17 Savannah Pooler Pky Tanger Outlets Savannah/ National 25 Wildlife Hilton Head Refuge Int'l Airport Bourne Ave A B (SAV)C D E F G H I HERTY AVE ROTHWELL ST BOU P RNE (ExitRO 104 GARDEN National MuseumBLV D 80 D U A 405 C AZALEA AVE T u A AVE Speedway Blvd W 26 of the Mighty SU HILL R g CITYMELI WH P IG D CA P r ATLE off PI-95)O D B u IP Y A K BLOOMINGDALE ST D R WINGS RD s e T R V ROMMEL RD IN V Q A N Eighth Air Force D R t R v L E W T D U a N L A i IN B R E S S S SAN DR E O V KIE Y R D D E R N D R R O N R D N E A E d E A O L LL A V t T I V E T M V h R S E E E E N V BRAMPTON RD ig L A I r L A E SOUTH K Y V d W D O ELI WHITNEY BLVD AW B A n H D C S l I H E L A L L a S Rogers St M G E a O S h n LEGEND r S SKINNER AVE 102 S a a Old River Rd d l RU Main St nn C CAROLINA i Pooler a 21 e n n a ATHILL RD v e DR 17 a E B a d N Ocial Visit 1 C Park P C H Y n IO Bloomingdale Rd ipem W CK S u T Savannah a M akers A DA n AD V D N 16 ISON E U JO Information a AVE 95 C FO H l H BELL ST N Centers N A ST 404 L R O E SHARON CT L N POOLER DJS WAY IE D I S WESTON WAY G R T S A D Parking K Y RE VD R D ISU 3 BL A LE R N VE Hutchinson Back River Daufuskie P PIN 307 R D LE A E BA S 8 AL P RRE Pooler Pky Louisville Rd 4 T RO N Island Island Jim Gillis Historic Savannah Pkway N RD TH TH LA Restrooms Pine Barren Rd S LA TH Pine Barren Rd OLD LOUISVILLE RD VE T W RO A P IS E A AV 80 V V RYANS WAY D E Pooler Cross Rd SHEFTALL RD FOX ST A Places of Interest AS R 8TH ST N HW D 80 E T Calibogue OOD 516 WELDON ST T S GODBEE -

Ancyronyx Reticulatus and A. Pulcherrimus, Two New Riffle Beetle Species from Borneo, and Discussion About Elmid Plastron Structures (Coleoptera: Elmidae)
Zootaxa 3760 (3): 383–395 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3760.3.5 http://zoobank.org/urn:lsid:zoobank.org:pub:0C122F35-D2F7-483C-AF12-BAB5B7F8682B Ancyronyx reticulatus and A. pulcherrimus, two new riffle beetle species from Borneo, and discussion about elmid plastron structures (Coleoptera: Elmidae) JÁN KODADA1, MANFRED A. JÄCH2 & FEDOR ČIAMPOR JR3,4 1Department of Zoology, Faculty of Natural Science, Comenius University, Mlynská dolina B-1, SK-842 15 Bratislava, Slovakia. E-mail: [email protected] 2Naturhistorisches Museum Wien, Burgring 7, A-1010 Wien, Austria. E-mail: [email protected] 3Institute of Zoology, Slovak Academy of Sciences, Dúbravská cesta 9, SK-845 06 Bratislava, Slovakia. E-mail: [email protected] 4Corresponding author Abstract Two new species of Ancyronyx Erichson, 1847 (Coleoptera: Elmidae) are described from Borneo: A. pulcherrimus (Bru- nei) and A. reticulatus (Sabah). Habitus views, illustrations of important characters as well as plastron structures of Ancy- ronyx reticulatus are presented and discussed. Key words: Coleoptera, Elmidae, Ancyronyx, new species, morphology, Borneo Introduction The first taxonomic review of Ancyronyx Erichson, 1847 was published almost 150 years after the description of the genus (Jäch 1994), followed by descriptions of several additional new species and descriptions of larvae (Jäch 2003, 2004; Freitag & Jäch 2007, Freitag & Balke 2011, Bian et al. 2012 and Freitag 2012). Based on several newly described species, especially from the Philippines, the morphological characteristics of the genus were modified and some important character states had to be added (see Freitag 2012). -

New Campaign Focuses on Industrial Stormwater Pollution
Summer 2013 A publication of Chattahoochee Riverkeeper (CRK) RiverCHAT www.chattahoochee.org NEW CAMPAIGN FOCUSES ON INDUSTRIAL STORMWATER POLLUTION ook no further than Proctor Creek, a general permit under the starting in downtown Atlanta, for the National Pollutant Discharge Limpact of industrial stormwater pollu- Elimination System (NPDES) tion on nearby streams and neighborhoods. every five years that sets Recently selected as one of 11 waterways in out requirements for best the nation to participate in the EPA’s Urban management practices, Waters Federal Partnership, this tributary inspections, water quality to the Chattahoochee has long been plagued monitoring and reporting. by polluted runoff from sources such as CRK participated as the sole landfills, auto salvage yards, chemical and environmental representa- concrete plants. tive in the stakeholder group that negotiated and helped Controlling industrial stormwater runoff is a strengthen the permit, which daunting challenge throughout the Chat- was issued in 2012. tahoochee River basin. Our watershed is home to thousands of industrial facilities With thousands of industrial that operate equipment and store materials facilities across the state CRK’s Jess Sterling samples runoff from an industrial site in the Proctor Creek watershed. outside, which are exposed to precipitation. currently managed by a single Clean Water Act, or that it may be failing to In fact, state and federal environmental state employee, EPD is woe- meet the terms of the permit. regulatory agencies have identified the fully understaffed to ensure compliance and pollutants of concern from more than 28 enforce the permit. CRK has been told that Since last fall, we have communicated with types of industrial activities. -

An Archaeological Survey at Oak Level Mound: Investigating Settlement Patterns and Intrasite Use During the Middle Mississippian Period (A.D
Georgia State University ScholarWorks @ Georgia State University Anthropology Theses Department of Anthropology Spring 5-11-2013 An Archaeological Survey at Oak Level Mound: Investigating Settlement Patterns and Intrasite Use During the Middle Mississippian Period (A.D. 1150-1350) Billy J. McCarley Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/anthro_theses Recommended Citation McCarley, Billy J., "An Archaeological Survey at Oak Level Mound: Investigating Settlement Patterns and Intrasite Use During the Middle Mississippian Period (A.D. 1150-1350)." Thesis, Georgia State University, 2013. https://scholarworks.gsu.edu/anthro_theses/72 This Thesis is brought to you for free and open access by the Department of Anthropology at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Anthropology Theses by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. AN ARCHAEOLOGICAL SURVEY AT OAK LEVEL MOUND: INVESTIGATING SETTLEMENT PATTERNS AND INTRASITE USE DURING THE MIDDLE MISSISSIPPIAN PERIOD (A.D. 1150–1350) by BILLY J. MCCARLEY Under the Direction of Jeffrey B. Glover, PhD ABSTRACT This study is about a Middle Mississippian (A.D. 1150-1350) burial mound site known as Oak Level Mound. Located in the back swamps of Bryan County, Georgia 2.4 km south of the Ogeechee River, the site is situated amongst Live Oak hammocks and palmettoes. The earthen architecture and material remains found at Oak Level Mound during the fall of 2012 and winter 2013 tell a tale of ancient people whose subsistence included oysters, snail, and nuts. Their daily practices are expressed in burial mounds and utilitarian and/or status goods, such as plain, cord-marked, and complicated-stamped pottery. -

Unknown Species and Larval Stages of Extremely Long-Legged Beetles Discovered by DNA Test 18 October 2011
Unknown species and larval stages of extremely long-legged beetles discovered by DNA test 18 October 2011 study was realized in cooperation with the Palawan Council for Sustainable Development and the De La Salle University Manila. The scientists conducting this study, Hendrik Freitag and Michael Balke, used mitochondrial DNA, which should be identical in all developmental stages in a species, to assign the previously unknown larval stages to adult imagines. This method was very useful because the outside appearance of immature and mature stages of holometabolous insects look completely different and would not allow an easy assignment to each other. The extremely long legs and cross-like elytral color patterns of Ancyronyx beetles remind of spiders. Credit: H. Freitag (2007) The unknown larval stages and a new species of the curious Spider Water Beetles were described after their assignment by DNA sequences. These taxonomic works are groundwork for the development of water quality bioindicator systems This is a Philippine Spider Water Beetle in frontal view. in the tropics. This study of the AQUA Palawana Credit: H. Freitag (2009) biodiversity program in the Philippines was published in the journal ZooKeys. The research program AQUA Palawana has been The studied insects of the genus Ancyronyx have exploring the unique freshwater biodiversity of the extremely long legs, often accompanied by an eye- Philippine Island and biosphere reserve of catching cross-like elytral colour pattern, so that Palawan for more than a decade. Scientists from they remind of spiders. In point of fact they are the Senckenberg Museum of Zoology Dresden and "Riffle Beetles" (Elmidae) that are able to breathe the Bavarian State Collections of Zoology in through a plastron, a microfilm of air around their Munich have now described larvae and a new body surface that is microscopically enlarged by species of the curious Spider Water Beetles setose structures. -

PROPOSED Atlantic Sturgeon Critical Habitat Rivers in the Southeast U.S. 30°N Florida 80°W 75°W Table 1
80°W 75°W Virginia Ü 0 25 50 100 150 200 Miles C1 North Carolina C2 C3 35°N 35°N CU1 C4 South Carolina Carolina DPS Units C1 Roanoke River, NC CU2 C5 C6 C2 Tar-Pamlico River, NC Georgia C3 Neuse River, NC C4 Cape Fear River, NC Northeast Cape Fear River, NC SA1 SAU1 CU1 Cape Fear River, NC C7 C5 Pee Dee River, SC Waccamaw River, SC SA2 South Atlantic DPS Units Bull Creek, SC SA3 SA1 Edisto River, SC C6 Black River, SC SA4 North Fork Edisto River, SC C7 Santee River, SC South Fork Edisto River, SC Rediversion Canal, SC SA5 North Edisto River, SC North Santee River, SC South Edisto River, SC South Santee River, SC SA2 Combahee River, SC Tailrace Canal-W Cooper River, SC Salkehatchie, River, SC Cooper River, SC SA6 SA3 Savannah River, SC/GA CU2 Wateree River, SC SAU1 Savannah River, SC/GA Congaree River, SC SA4 Ogeechee River, GA Broad River, SC SA5 Altamaha River, GA Santee River, above L Marion, SC Oconee River, GA Lake Marion, SC SA7 Ocmulgee River, GA Diversion Canal, SC SA6 Satilla River, GA Lake Moultrie, SC SA7 St. Marys, GA/FL Rediversion Canal, SC 30°N PROPOSED Atlantic Sturgeon Critical Habitat Rivers in the Southeast U.S. 30°N Florida 80°W 75°W Table 1. Proposed Critical Habitat Units and Extents of the Units. Critical Habitat Unit Name DPS Nomenclature Water Body State Upper extent River kilometers River miles Roanoke Carolina Unit 1 (C1) Roanoke River North Carolina Roanoke Rapids Dam 213 132 Tar ‐ Pamlico Carolina Unit 2 (C2) Tar ‐ Pamlico River North Carolina Rocky Mount Mill Pond Dam 199 124 Neuse Carolina Unit 3 (C3) Neuse River North Carolina Milburnie Dam 345 214 Cape Fear Carolina Unit 4 (C4) Cape Fear River North Carolina Lock and Dam #2 151 94 Northeast Cape Fear River North Carolina Upstream side of Rones Chapel Road Bridge 218 136 Cape Fear Unoccupied Carolina Unoccupied Unit 1 (CU1) Cape Fear River North Carolina Huske Lock and Dam (a.k.a.