Few Cav 1.3 Channels Regulate the Exocytosis of a Synaptic Vesicle At
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
4-Nervous-System-Structure-PPT.Pdf
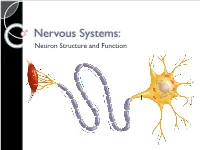
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation and coordination that produce coherency and result in harmonious function. Integration Cellular integration = processes within cells Whole-animal integration = selective combination and processing of sensory, endocrine, and central nervous system (CNS) information in ways that promote harmonious functioning of the whole organism within its environment. ◦ This includes its all its cells, tissues, and organs Integration Nerve cells are specialized for control and coordination. Integration ensures that an animal’s responses are smooth and coordinated rather than clashing or disjointed. Excitable Cells Neurons are a type of excitable cell ◦ Specially adapted to generate an electrical signal Can rapidly alter their membrane potential in response to an incoming signal. Vary in structure and function but use the same basic mechanism to send signals. Neuron Function – Main Points Specialized for processing and conveying information. Information is coded into changes in electrical potential across cell membranes. Neurons use action potentials to transmit these signals across long distances. Neurons allow animals to sense and respond to their environment. Benefits of Neurons Plants (no neurons): Action potentials travel @ 1-3 cm/sec Animals (neurons): Action potentials travel @ 100m/sec or 10,000cm/sec CNS to Muscles Signal Reception Dendrites & Cell Body Signal Integration Axon Hillock Signal Conduction Axon Signal Transmission Axon Terminals Signal Reception Dendrites sense and convert incoming signals into electrical signals by changing membrane potential. Cell Body = routine metabolic functions Signal Integration Incoming signals are conducted to the axon hillock If signal is sufficiently large an electrical signal, or action potential, is initiated. -

Topic 2 – Neuronal Physiology
Index Topic 2 – Neuronal Physiology - Resting membrane potential - The NA+-K+ pump - Channels - Graded potentials - Action potentials - Voltage-gated channels o Voltage-gated Na+ channel o Voltage-gated K+ channel § Repolarisation § Depolarisation - Propagation of action potentials Topic 3 – The Peripheral Nervous System - Efferent division o Somatic nervous system o Autonomic nervous system § Sympathetic nervous sysmte § Parasympathetic nervous system o Cholinergic neurons o Adrenergic neurons o Dominance o Rules about neurons o Neuromuscular junction o Comparison of a synapse and a neuromuscular junction Topic 4 – Skeletal Muscle - General points - Organisation of a muscle - Thick filaments - Thin filaments o Actin o Tropomyosin o Troponin - Transverse tubular system (t-tubules) - Sarcoplasmic reticulum - Contraction of a skeletal muscle fibre - Filament slide switch - Muscle twitches o Amount of fibres o Amount of force o Twitch response o Twitch summation o Tetanic contraction o ATP and skeletal muscle contraction o Direct phosphorylation o Oxidative phosphorylation o Glycolysis Topic 5 – Smooth Muscle - Similarities with skeletal muscle - Differences with skeletal muscle - Structure of smooth muscle - Dense bodies - Smooth muscle contraction ‘switch’ - Relaxation - Multi-unit smooth muscle - Single-unit smooth muscle o Pacemaker potentials o Slow-wave potentials Topic 6 – Gastrointestinal Physiology - Processes o Mobility o Secretion o Digestion o Absorption - Digestive system composition o List of organs o Importance of ‘separation’ -

Graded Potentials and Action Potentials
GRADED POTENTIALS AND ACTION POTENTIALS Near East University Faculty of Medicine Department of Biophysics Dr. Aslı AYKAÇ Nervous System Nervous system cells are comprised of glia and neurons. Neurons are responsible for receive, process, and transmit information in nervous system. • Glia – Not specialized for information transfer – Support neurons • Neurons (Nerve Cells) – Receive, process, and transmit information Information travels in one direction Dendrite → soma → axon Voltmetre (mV) 0 30 -30 50 -50 -70 outside ATP inside • All cells have electrical potential difference between inside and outside of the cell. • Transient changes in the membrane potential of its resting level produce electrical signals. – Such changes are the most important way that nerve cells process and transmit information. These signals occur in two forms: 1. graded potentials 2. action potentials Graded potentials are important in short distances. Action potentials are the long distance signals of nerve and muscle membranes. Nerve and muscle cells as well as some endocrine, immune, and reproductive cells have plasma membranes capable of producing action potentials. • These membranes – are called excitable membranes. – Their ability to generate action potentials is known as excitability. All cells are capable of conducting graded potentials, but excitable membranes can conduct action potentials. Changes in Membrane Potential depolarize The terms repolarize are used to describe hyperpolarize the direction of changes in the membrane potential relative to the resting potential. ) Membrane potential (mV potential Membrane Time The resting membrane potential (at -70 mV) is polarized. “Polarized” means that the outside and inside of a cell have a different net charge. • The membrane is said to be depolarized when its potential is less negative than the resting level. -

Lecture 7 Cell Signaling in the Nervous System All Body Cells Display a Membrane Potential, Which Is a Separation of Positive and Negative Charges Across the Membrane
Lecture 7 Cell signaling in the nervous system All body cells display a membrane potential, which is a separation of positive and negative charges across the membrane. This potential is related to the uneven distribution of Na, K, and large intracellular protein anions between the intracellular fluid (ICF) and extracellular fluid (ECF), and to the differential permeability of the plasma membrane to these ions. Membrane potential Types of changes in membrane potential are shown in Figure1: Figure 1 Types of changes in membrane potential. Electrical signals are produced by changes in ion movement across the plasma membrane Because the water-soluble ions responsible for carrying charge cannot penetrate the plasma membrane’s lipid bilayer, these charges can cross the membrane only through channels specific for them or by carrier-mediated transport. Membrane channels may be either leak channels or gated channels. As described in Chapter 3, leak channels, which are open all the time, permit unregulated leakage of their specific ion across the membrane through the channels .Gated channels, in contrast, have gates that can be open or closed, permitting ion passage through the channels when open and preventing ion passage through the channels when closed. Gate opening and closing results from a change in the conformation (shape) of the protein that forms the gated channel. There are four kinds of gated channels, depending on the factor that causes the change in channel conformation: (1) voltage-gated channels open or close in response to changes in membrane potential; (2) chemically gated channels change conformation in response to binding of a specific extracellular chemical messenger to a surface membrane receptor; (3) mechanically gated channels respond to stretching or other mechanical deformation; and (4) thermally gated channels respond to local changes in temperature (heat or cold). -

The Cells That Make Us Who We Are How Neurons Communicate With
Communication within the Nervous System The Cells that make us who we are How neurons communicate with one another Garrett: Brain & Behavior 4e 1 The Cells That Make Us Who We Are • How many are there? • Neurons: 100 billion • Make up 10% of brain volume • Glia: Many more! • Make up 90% of brain volume • Neurons: Jobs include • convey sensory information to the brain; • carry out operations involved in thought and feeling; • Send commands out to the body. • Dendrites • Cell body or soma Garrett: Brain & Behavior 4e • Axons insulated with myelin (secreted by glia), with end terminals that release neurotransmitters from vesicles into the synapse 2 The Cells That Make Us Who We Are Figure 2.3: Components of a Neuron Garrett: Brain & Behavior 4e 3 The Cells That Make Us Who We Are Figure 2.4 a,b : The Three Shapes of Neurons • Unipolar neurons (a) • Bipolar neurons (b) • Multipolar neurons • Figure 2 .3, previous slide Garrett: Brain & Behavior 4e 4 The Cells That Make Us Who We Are Table 2.1: The Three Types of Neurons Figure 2.4c: The Three Shapes of Neurons Type Shape Description Motor neuron Multipolar Output to muscles/organs Sensory neuron Unipolar or Bipolar Input from receptors Interneuron Multipolar Most within the CNS. Most common. Garrett: Brain & Behavior 4e 5 The Cells That Make Us Who We Are Figure 2.5: Composition of the Cell Membrane • Lipids • Heads attracted to water in and outside the cell, tails repelled by water • Creates a double-layer membrane • Proteins • Hold the cells together • Controls the environment in and around the cell Garrett: Brain & Behavior 4e 6 The Neural Membrane • The neuron has a selectively-permeable membrane. -

Resting Membrane Potential
Chapter 06 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright ©2017 McGraw-Hill Education. Permission required for reproduction or display. 1 Topics Section A Cells of the nervous Section C Synapses system 6.8 Functional anatomy of synapses 6.1 Structure and maintenance of 6.9 Mechanisms of neurotransmitter neurons release 6.2 Functional classes of neurons 6.10 Activation of the postsynaptic cell 6.3 Glial cells 6.11 Synaptic integration 6.4 Neural growth and regeneration 6.12 Synaptic strength Section B Membrane Potentials 6.13 Neurotransmitters and 6.5 Basic principles of electricity neuromodulators 6.6 The resting membrane potential 6.14 Neuroeffector communication 6.7 Graded potentials and action Section D Structure of the nervous potentials system 6.15 Central nervous system: Brain 6.16 Central nervous system: Spinal cord 6.17 Peripheral nervous system 6.18 Autonomic nervous system 6.19 Protective elements associated 2 with the brain The Nervous System • The Nervous System has two major divisions: – The Central Nervous System (CNS), which is composed of the brain and spinal cord. – The Peripheral Nervous System (PNS) is composed of the nerves that connect the brain or spinal cord with the body’s muscles, glands, and sense organs. • The neuron is the basic cell type of both systems. 3 Structure of a Neuron 4 Schwann Cells and Myelin • Schwann cells surround and form myelin sheaths around the larger nerve fibers. These are vital to regeneration and proper nerve signal conduction. 5 Myelination of Axons 6 Axonal Transport 7 Functional Classes of Neurons 8 Functional Classes of Neurons 9 10 Synapses Synapses can use both chemical and electrical stimuli to pass information. -

Week 05 Assignment
Week 05 Assignment 1. Gated channels that open or close in response to a change in the transmembrane potential are a. mechanically regulated channels b. voltage regulated channels c. chemically regulated channels d. all options are correct Ans: B 2. Changes in transmembrane potential that are not restricted to the area surrounding the site of simulation are a. action potentials b. graded potentials c. inhibitory potentials d. hyperpolarizing potentials Ans: A 3. The synapsing of several neurons on the same postsynaptic neuron is a. serial processing b. reverberation c. divergence d. convergence Ans: D 4. If the resting membrane potential is -70mV and the threshold is -55mV, a membrane potential of -60mV will a. produce an action potential b. make it easier to produce an action potential c. make it harder to produce an action potential d. hyperpolarize the membrane Ans: B 5. A graded potential a. decrease with distance from the point of stimulation b. spreads passively because of local currents c. may involve either depolarization or hyperpolarization d. all options are correct Ans: D 6. For an action potential to begin, an area of excitable membrane must a. have its voltage-regulated gates inactivated b. be hyperpolarized c. be depolarized to threshold level d. not be in relative refractory period Ans: C 7. During an absolute refractory period, the membrane a. continues to hyperpolarize b. cannot respond to further stimulation c. can respond to a larger-than-normal depolarizing stimulus d. will respond to summated stimulation Ans: B 8. Action potentials are restricted to areas of excitable membranes that contain voltage regulate channels a. -

Communication: Chemical and Electrical Signaling
4 Communication: Chemical and Electrical Signaling Major Themes Cell-to-cell communication is critical for homeostasis and life. Communication requires a sender, a signal, a medium to carry the signal, and a receiver to accept the signal. Signals must be “translated” from a code into action. The effect of the signal is determined by the receiver, not merely by the signal. There are two kinds of physiological signals: chemical and electrical. 4. Explain why every cell may encounter a Chapter Objectives chemical signal, but not every cell responds to the chemical signal. The Nature of Communication 112 5. List the steps involved in signaling by 1. Identify the four components required for lipophilic signal molecules at intracellular communication. receptors. Chemical Signaling 113 Electrical Signaling 120 2. Explain the difference between paracrine 6. Defi ne membrane potential, and explain factors, hormones, neurotransmitters, and the difference between a membrane neurohormones. potential of –100 mV and a membrane 3. Name three types of cell membrane potential of ϩ30 mV. receptors, and explain how they enable 7. Explain why the resting membrane signaling molecules to affect intracellular potential is negative. events without entering the cell. LWBK585-c04_p110-137.indd 110 8/2/10 4:40:01 PM 8. Sketch a neuron, labeling the cell body, dendrites, Caffeine and Communication: The Case axon, and myelin. of Andy M. 132 9. Compare and contrast action potentials and 13. Explain why Andy’s synaptic activity is reduced graded potentials. when he drinks decaffeinated coffee. In your 10. Name the four phases of the action potential, and explanation, use the following terms (not discuss the involvement of sodium and/or necessarily in order): caffeine, adenosine, fi rst and potassium channels in each phase. -

Nervous Tissue
11 Nervous Tissue Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Cellular Organization of the Nervous System Learning Outcomes 11.1 Describe the anatomical and functional divisions of the nervous system. 11.2 Sketch and label the structure of a typical neuron, and describe the functions of each component. 11.3 Classify and describe neurons on the basis of their structure and function. © 2018 Pearson Education, Inc. Section 1: Cellular Organization of the Nervous System Learning Outcomes (continued) 11.5 Describe the locations and functions of Schwann cells and satellite cells. 11.4 Describe the locations and functions of neuroglia in the CNS. © 2018 Pearson Education, Inc. Module 11.1: The nervous system has three divisions: the CNS, PNS, and ENS Nervous system—three divisions 1. Central nervous system (CNS) • Brain and spinal cord • Information processing—integrates, processes, coordinates sensory and motor commands 2. Peripheral nervous system (PNS) • All nervous tissue outside CNS, excluding the ENS 3. Enteric nervous system (ENS) • Neural tissues in wall of gastrointestinal tract; helps control digestive function © 2018 Pearson Education, Inc. Module 11.1: Nervous system divisions Subdivisions of the peripheral nervous system . Sensory (afferent) division brings information to CNS from receptors in peripheral tissues and organs • Sensory receptors – Position, touch, pressure, pain, temperature • Special sensory organs – Smell, taste, sight, balance, hearing © 2018 Pearson Education, Inc. Module 11.1: Nervous system divisions . Motor (efferent) division carries motor commands from CNS • Somatic nervous system (SNS) – Voluntary nervous system—conscious control of movement – To skeletal muscles; conscious control of movement • Autonomic nervous system (ANS) – Involuntary nervous system—automatically regulates activities – To smooth muscle, cardiac muscle, glands, adipose tissue © 2018 Pearson Education, Inc. -

Coding with Spike Shapes and Graded Potentials in Cortical Networks Mikko Juusola,1* Hugh P.C
Problems and paradigms Coding with spike shapes and graded potentials in cortical networks Mikko Juusola,1* Hugh P.C. Robinson,2 and Gonzalo G. de Polavieja3* Summary into digital patterns of spikes (Fig. 1A). The carriers of In cortical neurones, analogue dendritic potentials are information are then the temporal patterns of spikes or even thought to be encoded into patterns of digital spikes. According to this view, neuronal codes and computa- a simpler abstraction like the spike rate (Fig. 1B). We refer to tions are based on the temporal patterns of spikes: spike this as the ‘pulse code’. At the other end of the spectrum of times, bursts or spike rates. Recently, we proposed an possibilities is the view in which all mechanisms affecting ‘action potential waveform code’ for cortical pyramidal synaptic integration and transmission are relevant in the neurones in which the spike shape carries information. neural code (Fig. 1C). Here, we argue that the latest Broader somatic action potentials are reliably produced in response to higher conductance input, allowing for experimental evidence points to an intermediate situation in four times more information transfer than spike times which some other features of the membrane potential alone. This information is preserved during synaptic trajectory (Fig. 1D), apart from the sequence of spike times, integration in a single neurone, as back-propagating significantly influence synaptic output (Fig. 1E). action potentials of diverse shapes differentially shunt Our recent work(1) established that the different action incoming postsynaptic potentials and so participate in the next round of spike generation. An open question has potential (AP) shapes produced by the same pyramidal been whether the information in action potential wave- cortical neurone are not random but correspond to the level forms can also survive axonal conduction and directly of the previous conductance. -

Teaching Principles of Chemical Synaptic Transmission Without Action Potentials
The Journal of Undergraduate Neuroscience Education (JUNE), Spring 2021, 19(2):R28-R30 AMAZING PAPERS IN NEUROSCIENCE Getting Graded: Teaching Principles of Chemical Synaptic Transmission Without Action Potentials Laura Strathern School of Psychology and Neuroscience, University of St Andrews, St Andrews, UK KY16 9JP. The study of synaptic transmission is foundational for depolarizations in presynaptic neurons. This was one of the undergraduate neuroscience students. Chemical synaptic first papers to clearly demonstrate that both graded and transmitter release is usually presented as evoked by action spike-mediated chemical synaptic transmission could occur potentials, but it can also be caused by subthreshold, concurrently at an identified synapse. Discussion of this ‘graded’ changes in presynaptic membrane potential. In a work in undergraduate classrooms can encourage students 1980 publication in the Proceedings of the National to think in greater depth about the diversity of transmission Academy of Sciences, Graubard and colleagues measured mechanisms available to neurons. synaptic activity in the crustacean stomatogastric ganglion; they found that postsynaptic voltage changes occur in Key words: action potential; graded chemical synaptic response to both action potentials and to graded transmission; stomatogastric ganglion; electrophysiology Chemical synaptic transmission between neurons requires Therefore, teaching this type of synaptic transmission to the release of neurotransmitter molecules from a undergraduate neuroscience students can expand presynaptic neuron into a synaptic cleft. Once released, understanding of the diversity of synaptic transmission neurotransmitters bind to receptors on the membranes of mechanisms. postsynaptic neurons. Action-potential-evoked release is common within the nervous system. In this mode, an action RESEARCH SUMMARY potential is generated and travels along the axon to the Graubard et al. -

Nerve Cell Physiology Resting Potential
Nerve Cell Physiology body is electrically neutral there are small differences in electrical charge between inside and outside of cell membranes due to differences in + and – ions on inside and outside of cell membrane differences in charge = potential (stored energy) potential difference is measured as voltage for body cells voltage is measured in millivolts 1mV = 0.001 V resting cells (all cells in body) have potential difference = -50 to –200 mV muscle and nerve cells –40 to –90 mV average: -70 mV the flow of charge = current in wires e- flow to produce current = electrical in neurons ions flow to produce current = electrochemical all cells have ≠ charges on each side of membrane only nerve and muscle cell membranes can rapidly alter this charge by changing permeability of the membrane Resting Potential resting membrane potentials of neurons and muscle cells are created & maintained by: 1. There are different kinds and concentrations of ions inside and outside cell membranes: Inside negative ions: proteins, PO4---, SO4-- cell positive ions: K+ Outside negative ions: HCO3-, Cl- cell positive ions: Na+ 1 2. there are more negative ions inside cell especially proteins negative ions generally can’t cross membrane 3. Active transport maintains concentration gradients of some of these + ions: more Na+ outside cell more K+ inside cell Sodium/Potassium pump pumps Na+ out and K+ in but pumps 3 Na+ out for every 2 K+ in 4. cell membrane is permeable to both Na+ and K+ by passive (leakage) channels but more permeable to K+ than to Na+ (50-100x’s