LAP-BAND AP® Adjustable Gastric Banding System with Rapidport® EZ and OMNIFORM™ Design
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Continent Urostomy Guide
$POUJOFOU6SPTUPNZ(VJEF "QVCMJDBUJPOPGUIF6OJUFE0TUPNZ"TTPDJBUJPOTPG"NFSJDB *OD i4FJ[FUIF 0QQPSUVOJUZw CONTINENT UROSTOMY GUIDE Ilene Fleischer, MSN, RN, CWOCN, Author Patti Wise, BSN, RN, CWOCN, Author Reviewed by: Authors and Victoria A.Weaver, RN, MSN, CETN Revised 2009 by Barbara J. Hocevar, BSN,RN,CWOCN, Manager, ET/WOC Nursing, Cleveland Clinic © 1985 Ilene Fleischer and Patti Wise This guidebook is available for free, in electronic form, from United Ostomy Associations of America (UOAA). UOAA may be contacted at: www.ostomy.org • [email protected] • 800-826-0826 CONTENTS INTRODUCTION . 3 WHAT IS A CONTINENT UROSTOMY? . 4 THE URINARY TRACT . 4 BEFORE THE SURGERY . .5 THE SURGERY . .5 THE STOMA . 7 AFTER THE SURGERY . 7 Irrigation of the catheter(s) 8 Care of the drainage receptacles 9 Care of the stoma 9 Other important information 10 ROUTINE CARE AT HOME . 10 Catheterization schedule 11 How to catheterize your pouch 11 Special considerations when catheterizing 11 Care of the catheter 12 Other routine care 12 HELPFUL HINTS . .13 SUPPLIES FOR YOUR CONTINENT UROSTOMY . 14 LIFE WITH YOUR CONTINENT UROSTOMY . 15 Clothing 15 Diet 15 Activity and exercise 15 Work 16 Travel 16 Telling others 17 Social relationships 17 Sexual relations and intimacy 17 RESOURCES . .19 GLOSSARY OF TERMS . 20 BIBLIOGRAPHY . .21 1 INTRODUCTION Many people have ostomies and lead full and active lives. Ostomy surgery is the main treatment for bypassing or replacing intestinal or urinary organs that have become diseased or dysfunctional. “Ostomy” means opening. It refers to a number of ways that bodily wastes are re-routed from your body. A urostomy specifi cally redirects urine. -

A Practical Guide for Stoma Problems Developed by the Ostomy Forum
A practical guide for Stoma problems Developed by the Ostomy Forum Dedicated to Stoma Care A practical guide for Stoma and Peristomal skin problems A practical guide for Stoma Developed by: Frances McKenzie, Amanda Smith, Doreen Woolley, Beverley Colton, Bart Tappe and Global Clinical Marketing, Dansac A/S. The practical guide is based on the Observation Index developed by the Ostomy Forum group (a specialized group of ET nurses from Sweden, Norway, The Netherlands, Poland, Japan, UK and Denmark) and is Normal Stoma made to help you manage common stoma and peristomal skin problems you might come across in your nursing practice. Stoma is a Greek word that means opening or mouth. It is a surgically created opening that can be temporary or permanent and allows for the Sharing best practice by use of this educational tool will lead to early excretion of faecal waste (colostomy, ileostomy) or urine (urostomy). detection and appropriate intervention to secure a high standard of stoma care. A stoma is a surgically made opening of the bowel: • The bowel is brought out through the abdominal wall This tool should be used in consultation with your Stoma Care Specialist. • It is matured and sutured subcutaneously • Faeces and urine will pass and be collected in a specially designed Disclaimer: ostomy pouch. We recognize that nurses in other practices will have different ways of treating the identified problems. The scope of this guide is to give first In the following pages you will find examples of different stoma problems step, easy to use, practical advice that is recognized and accepted and concrete suggestions for intervention and management of the stoma. -

Adjustable Gastric Banding
7 Review Article Page 1 of 7 Adjustable gastric banding Emre Gundogdu, Munevver Moran Department of Surgery, Medical School, Istinye University, Istanbul, Turkey Contributions: (I) Conception and design: All authors; (II) Administrative support: All authors; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Emre Gündoğdu, MD, FEBS. Assistant Professor of Surgery, Department of Surgery, Medical School, Istinye University, Istanbul, Turkey. Email: [email protected]; [email protected]. Abstract: Gastric banding is based on the principle of forming a small volume pouch near the stomach by wrapping the fundus with various synthetic grafts. The main purpose is to limit oral intake. Due to the fact that it is a reversible surgery, ease of application and early results, the adjustable gastric band (AGB) operation has become common practice for the last 20 years. Many studies have shown that the effectiveness of LAGB has comparable results with other procedures in providing weight loss. Early studies have shown that short term complications after LAGB are particularly low when compared to the other complicated procedures. Even compared to RYGB and LSG, short-term results of LAGB have been shown to be significantly superior. However, as long-term results began to emerge, such as failure in weight loss, increased weight regain and long-term complication rates, interest in the procedure disappeared. The rate of revisional operations after LAGB is rapidly increasing today and many surgeons prefer to convert it to another bariatric procedure, such as RYGB or LSG, for revision surgery in patients with band removed after LAGB. -
Hints and Tips
Hernia Simon, colostomy since 2010 Hints & Tips Dedicated to Stoma Care Dedicated to Stoma Care FOREWORD & ACKNOWLEDGEMENTS This booklet offers guidance to the person undergoing surgery which will result in stoma formation or for those post-operatively who may be at risk of or perhaps already have developed a parastomal hernia. Please discuss the content with your Stomal Therapy Nurse (STN) if you require additional advice or support. CONTENT What is a parastomal hernia? .........................................3 Am I at risk of developing a parastomal hernia? ������������4 What is my ideal weight? ................................................5 Practical hints & tips to reduce risk of developing a parastomal hernia .....................................6 I think I’ve developed a hernia - what should I do? ���������7 Parastomal hernia management �������������������������������������8 Exercise ..........................................................................9 Dansac would like to thank the following for their invaluable contribution to this booklet: Sharon Colman BSc (Hons) Community Stoma Nurse Specialist, Norfolk. Kevin Hayles Dip HE, RN Queens Hospital Romford, Essex. Debbie Johnson RGN, Stoma Specialist, Dansac UK, London Community. Jacqui North RGN BSc(Hons), Senior Clinical Nurse Specialist, Stoma Care, SE London Community. Jo Sica Clinical Nurse Specialist, Stoma Care, Kingston CCG. 2 WHAT IS A PARASTOMAL HERNIA? Parastomal hernia is a common complication which can affect some people following stoma formation. Research has shown that as many as 10-50% of patients may go on to develop a hernia.6, 9, 10 During your surgery an incision is made through the abdominal wall and muscle. This can result in a weakness in the muscle surrounding your stoma which may lead to a noticeable bulge behind or around the stoma. -

“Eating with an Ostomy” Nutrition Guide
1 EATING WITH AN OSTOMY A Comprehensive Nutrition Guide for Those Living with an Ostomy First Edition by Joanna Burgess-Stocks BSN, RN, CWOCN A publication of UOAA, United Ostomy Associations of America 2 The printing of this publication was made possible by generous contributions from Sherry Lessard, George & Linda Salamy and the San Francisco (Golden Gate) Affiliated Support Group. Copyright © 2020 UOAA. All Rights Reserved. Disclaimer: This document contains information developed by United Ostomy Associations of America. This information does not replace medical advice from your health care provider. You are a unique individual and your experiences may differ from that of other patients. Talk to your health care provider if you have any questions about this document, your condition, or your treatment plan. Table of Contents 4 Acknowledgements 7 Introduction 9 The Role of the Registered Dietitian 11 Nutrition 101—The Basics 20 Ostomy and the Digestive System 26 Ostomy and the Urinary System 31 Post-Operative Nutritional Guidelines: The First 4–6 Weeks 35 Ileostomy: Specific Post-Op Guidelines 38 Nutrition after Recovery and Beyond 41 Hydration, Fluids, and Electrolytes 45 Ostomy and Medications 52 Guidelines for a Continent Fecal Diversion 55 Short Bowel Syndrome 60 Resources 63 Glossary of Terms 70 Appendix: Food Journal Food and Their Effects Chart References Testimonials Acknowledgements Thank you to all who worked diligently in the creation of this nutrition guide for people living with or facing ostomy surgery. This document came to fruition with the help and expertise of registered dietitians, wound ostomy and continence nurses, medical educators, and patient reviewers. -

Intestinal Stomas Proximal the Ileostomy
INTESTINAL total water and sodium in the body. This is accentuated the more Intestinal stomas proximal the ileostomy. In the early stages, a distal ileostomy typically discharges 1000–2000 ml/day, but can increase to Richard N Saunders 5000–6000 ml/day in exceptional circumstances, leading to dehy- David Hemingway dration and electrolyte abnormalities. Occasionally, this is driven by proximal disease (e.g. Crohn’s disease), but a cause is not identified in many cases. The output can usually be reduced to 250–750 ml/day with supportive care and antidiarrhoeal agents. In the longer term, the systemic effects of an ileostomy include an increased incidence of renal tract calculi (60% composed of uric acid). Some studies also suggest a threefold increase in the incidence of gallstones, but this remains controversial. Abstract Intestinal stomas are frequently necessary after abdominal surgery. A Principles of stoma formation thorough understanding of this less than glamorous area of surgical practice can prevent complications and make a significant difference Discussion – the possibility of a stoma should be discussed to patients. The first part of this review focuses on the basic principles with patients undergoing elective or emergency colorectal sur- underpinning the management of intestinal stomas (i.e. physiology, for- gery. A Stoma Nurse should be involved as early as possible. The mation, common complications). The second part comments briefly on location and construction of the stoma is critical in ensuring good specific types of stoma and their role in current clinical practice. quality of life and avoiding management problems. Assessment – preoperatively, patients are assessed lying Keywords colostomy; ileostomy; intestinal surgery; stoma down, sitting and standing, and the best sites marked. -

Pdfs–For–Download/Ostomy–Care/Whats–Right–For– Me–-–Ileostomy 907602-806.Pdf on October 2, 2019
cancer.org | 1.800.227.2345 Ileostomy Guide Ileostomy surgery is done for many different diseases and problems. Some conditions that can lead to ileostomy surgery include ulcerative colitis, Crohn’s disease, familial polyposis, and cancer. Sometimes an ileostomy is only needed for a short time (temporary), or it may be needed for the rest of a person's life (permanent). For the thousands of people who have serious digestive diseases, an ileostomy can be the start of a new and healthier life. If you’ve had a chronic (long-term) problem or a life- threatening disease like cancer, you can look forward to feeling better after you recover from ileostomy surgery. You can also look forward to returning to most, if not all of the activities you enjoyed in the past. This guide will help you better understand ileostomy – what it is, why it’s needed, how it affects the normal digestive system1, and what changes it brings to a person’s life. ● What Is an Ileostomy? ● Types of Ileostomies and Pouching Systems ● Caring for an Ileostomy What Is an Ileostomy? An ileostomy is an opening in the belly (abdominal wall) that’s made during surgery. It's usually needed because a problem is causing the ileum to not work properly, or a disease is affecting that part of the colon and it needs to be removed. The end of the ileum (the lowest part of the small intestine) is brought through this opening to form a 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 stoma, usually on the lower right side of the abdomen. -

Continent Urinary Reservoirs and Bladder Substitutes.Pdf
������������������ ��������������� ������������������� �������������������� ���������������� ��������������������� Continent Cutaneous Urinary Reservoirs and Bladder Substitutes Anatomy The bladder is an organ in the pelvis that collects, stores and expels urine. Urine is produced by the kidneys and travels down two tube-like structures called the ureters. The ureters connect the kidneys to the bladder. Urine leaves the bladder through another tube-like structure called the urethra. (Figure 1) Removal of the bladder (cystectomy) may be necessary in some people with bladder cancer, congenital disor- ders of the urinary tract, and in some people who have suffered surgical, traumatic or neurologic damage to the bladder. In these situations, another method of col- lecting and excreting urine must be found. The most common and easiest method for urinary diversion is to use a short piece of intestine as the connection between the ureters and the outside of the body (ileal or colon conduit). This type of diversion is easy for the patient to manage and has a low rate of complication. However, an ostomy bag must be worn at all times to collect urine. Newer surgical techniques are available which do not require the patient to wear an ostomy bag. These newer proce- dures involve creation of a continent urinary reser- voir that collects and stores urine. What is a Continent Urinary Reservoir and How is it Made? A continent urinary reser- voir is an internal “pouch” made from segments of the intestine. Urinary reservoirs can be made from small intestine alone, large intestine alone or from a combination of the above. (Figure 2) The bowel segments selected for use are disconnected from the remainder of the intestinal tract to avoid mixing the gastrointestinal contents (feces) with urine. -
Abdominoperineal Excision of the Rectum Information
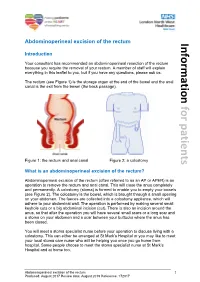
Abdominoperineal excision of the rectum Information Introduction Your consultant has recommended an abdominoperineal resection of the rectum because you require the removal of your rectum. A member of staff will explain everything in this leaflet to you, but if you have any questions, please ask us. The rectum (see Figure 1) is the storage organ at the end of the bowel and the anal canal is the exit from the bowel (the back passage). for patients Figure 1: the rectum and anal canal Figure 2: a colostomy What is an abdominoperineal excision of the rectum? Abdominoperineal excision of the rectum (often referred to as an AP or APER) is an operation to remove the rectum and anal canal. This will close the anus completely and permanently. A colostomy (stoma) is formed to enable you to empty your bowels (see Figure 2). The colostomy is the bowel, which is brought through a small opening on your abdomen. The faeces are collected into a colostomy appliance, which will adhere to your abdominal wall. The operation is performed by making several small keyhole cuts or a big abdominal incision (cut). There is also an incision around the anus, so that after the operation you will have several small scars or a long scar and a stoma on your abdomen and a scar between your buttocks where the anus has been closed. You will meet a stoma specialist nurse before your operation to discuss living with a colostomy. This can either be arranged at St Mark’s Hospital or you may like to meet your local stoma care nurse who will be helping you once you go home from hospital. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

Ileostomy Guide
Ileostomy Guide A Publication of United Ostomy Associations of America, Inc. A Message To You... Ileostomy surgery is a lifesaving surgery that enables a person to enjoy a full range of activities, including traveling, sports, family life and work. Thousands of people annually undergo ostomy surgery for various reasons and return to a healthy, functioning lifestyle. The United Ostomy Associations of America (UOAA) is a volunteer organization dedicated to helping those who have or will have ostomy or other diversionary surgery by providing one-on-one support, local support group meetings, conferences, and educational material through its web site, printed material and The Phoenix magazine. You have many peers in the UOAA who are ready to answer your questions, provide support and reassure you that you can have a full, productive life after ostomy surgery. We invite you to join us as we fulfill our mission in helping others. From the United Ostomy Associations of America ILEOSTOMY GUIDE Reviewed by: Barbara Morrow, RN, MSN, CWOCN 2017 Nancy Gutman, RN, CWOCN 2011 This guidebook is available for free, in electronic form, from the United Ostomy Associations of America (UOAA). It was originally produced, copyrighted and sold by the United Ostomy Association (UOA), the national US ostomy organization from 1962 to 2005, which released its copyrights on this material. UOAA may be contacted at: www.ostomy.org • [email protected] • 800-826-0826 CONTENTS INTRODUCTION . 6 FACTS ABOUT ILEOSTOMIES . 7 NORMAL DIGESTIVE SYSTEM. 8 Small intestine. 8 Large intestine . 8 TYPES OF SMALL BOWEL DIVERSION . 9 Ileoanal reservoir (J-Pouch) . .9 Brooke ileostomy . -

Percutaneous Endoscopic Gastrostomy Tube-Associated Metastasis in Pharyngo- Oesophageal Malignancy
RCSIsmjcase report Percutaneous endoscopic gastrostomy tube-associated metastasis in pharyngo- oesophageal malignancy Royal College of Surgeons in Ireland Student Medical Journal 2012; 5: 54-57. Introduction pharyngo-oesophageal carcinoma.8 On average, A large proportion of patients with 10,000 PEG tubes per year are placed in patients pharyngo-oesophageal carcinoma will either with pharyngo-oesophageal malignancy in the present with or develop dysphagia and United States. This statistic, along with a mean odynophagia due to iatrogenic intervention or survival time of only 4.3 months in confirmed as a result of advancing disease. Gastrostomy cases of metastasis, illustrates the importance of tubes are an indispensable resource for determining the exact relationship between PEG maintaining nutrient intake in patients who are placement and stomal recurrence.1,9 Knowledge unable to take in adequate oral nutrition.1-5 of the incidence, pathophysiology, clinical The optimum technique of gastrostomy relevance and prevention measures of placement in the setting of head and neck PEG-associated stomal metastasis in head and malignancy remains controversial. Currently, the neck cancer patients remains extremely limited. most commonly used method of establishing enteral feeding in this patient subset is the Case report minimally invasive ‘pull’ percutaneous A 60-year-old male presented to his GP with endoscopic gastrostomy (PEG) technique, first persistent odynophagia. After several failed described by Gauderer et al. in 1980.2,3 In courses of antibiotics, the patient was referred Matthew Hearn1, addition to the usual complications of PEG tube to an otolaryngology clinic for further Brent Trull1, insertion, patients undergoing this procedure for investigation.