Opportunities and Challenges in the Delivery of Mrna-Based Vaccines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rnai in Primary Cells and Difficult-To-Transfect Cell Lines
Automated High Throughput Nucleofection® RNAi in Primary Cells and Difficult-to-Transfect Cell Lines Claudia Merz, Bayer Schering Pharma AG, Berlin, Germany; Andreas Schroers, amaxa AG, Cologne, Germany; Eric Willimann, Tecan AG, Männedorf, Switzerland. Introduction Materials & Methods - Workflow Using primary cells for RNAi based applications such as target identification or – validation, requires a highly efficient transfection displaying the essential steps of the automated Nucleofector® Process: technology in combination with a reliable and robust automation system. To accomplish these requirements we integrated the amaxa 1. Transfer of the cells to the Nucleocuvette™ plate, 96-well Shuttle® in a Tecan Freedom EVO® cell transfection workstation which is based on Tecan’s Freedom EVO® liquid handling 2. Addition of the siRNA, (Steps 1 and 2 could be exchanged), platform and include all the necessary components and features for unattended cell transfection. 3. Nucleofection® process, 4. Addition of medium, Count Cells 5. Transfer of transfected cells to cell culture plate for incubation ® Nucleofector Technology prior to analysis. Remove Medium The 96-well Shuttle® combines high-throughput compatibility with the Nucleofector® Technology, which is a non-viral transfection method ideally suited for primary cells and hard-to-transfect cell lines based on a combination of buffers and electrical parameters. Nucleocuvette Plate Add Nucleofector +– The basic principle and benefits of the (empty) Solution Cell of interest Gene of interest Nucleofector® -

Xfect™ RNA Transfection Reagent Protocol-At-A-Glance
Xfect™ RNA Transfection Reagent Protocol-At-A-Glance I. Introduction A. Summary This Protocol-At-A-Glance is provided for transfection of cells with RNA using the Xfect RNA Transfection Reagent (Cat. No. 631450). It describes transfection of mammalian cells with RNA (mRNA, sgRNA, microRNA, or shRNA) in a 12-well plate format. For formats other than 12-well plates, see Tables I and II for appropriate reaction volumes. Transfections can be carried out entirely in the presence of serum. The protocol is divided into two sections: Section II describes transfection of cells with RNA only, without the cotransfection of DNA. Section III describes cotransfection of cells with both RNA and DNA. NOTE: When transfecting cells with mRNA, expression may be increased by using serum-free medium during transfection, since serum may contain RNases that can reduce the amount of full-length mRNA. B. General Considerations Storage & handling Store the Xfect RNA Transfection Polymer at –20°C. Do not thaw until ready to use. Once thawed, store at 4°C for up to 12 months. NOTE: The Xfect RNA Transfection Polymer is a milky suspension and should be vortexed briefly prior to use to ensure that it is fully resuspended. Thaw Xfect Reaction Buffer at room temperature just prior to use. Vortex after thawing. Once thawed, store Xfect Reaction Buffer at 4°C for up to 12 months. Xfect Polymer The protocol for cotransfection with DNA (Section III) requires the use of the Xfect Polymer (not included; sold as part of the DNA Xfect Transfection Reagent, Cat. Nos. 631317, 631318). -

Updated May 26, 2021 Cross-Border Industry Partnerships on COVID-19 Vaccines and Therapeutics Vaccines • Curevac O Celonic Wi
Updated May 26, 2021 Cross-Border Industry Partnerships on COVID-19 Vaccines and Therapeutics Vaccines • CureVac o Celonic will manufacture 100 million doses of CureVac’s vaccine at its plant in Heidelberg, Germany, providing bulk substance for 50 million doses by the end of 2021. (press release) o Novartis will manufacture CureVac’s vaccine. (press release) o GlaxoSmithKline plc and CureVac N.V. announced a new €150m collaboration, building on their existing relationship, to jointly develop next generation mRNA vaccines for COVID-19 with the potential for a multi-valent approach to address multiple emerging variants in one vaccine. (press release) o Rentschler Biopharma SE will manufacture CureVac’s vaccine. (press release) o Bayer will support the further development, supply and key territory operations of CureVac’s vaccine candidate. (press release) o Fareva will dedicate a manufacturing plant in France to the fill and finish of CureVac’s vaccine. (press release) o Wacker Chemie AG will manufacture CureVac’s vaccine candidate at its Amsterdam site. (press release) o CureVac will collaborate with Tesla Grohmann Automation to develop an RNA printer that works like a mini-factory and can produce such drugs automatically. (press release) • Moderna o Samsung Biologics will provide large scale, commercial fill-finish manufacturing for Moderna’s vaccine in South Korea. (press release) o Baxter International will provide fill/finish services and supply packaging for Moderna. (press release) o Sanofi will manufacture 200 million doses of Moderna’s COVID-19 vaccine starting in September 2021. (press release) o Rovi will produce bulk substance for Moderna’s COVID-19 vaccine, expanding an agreement between the companies. -

Split Deoxyribozyme Probe for Efficient Detection of Highly Structured RNA Targets
University of Central Florida STARS Honors Undergraduate Theses UCF Theses and Dissertations 2018 Split Deoxyribozyme Probe For Efficient Detection of Highly Structured RNA Targets Sheila Raquel Solarez University of Central Florida Part of the Biochemistry Commons, and the Biology Commons Find similar works at: https://stars.library.ucf.edu/honorstheses University of Central Florida Libraries http://library.ucf.edu This Open Access is brought to you for free and open access by the UCF Theses and Dissertations at STARS. It has been accepted for inclusion in Honors Undergraduate Theses by an authorized administrator of STARS. For more information, please contact [email protected]. Recommended Citation Solarez, Sheila Raquel, "Split Deoxyribozyme Probe For Efficient Detection of Highly Structured RNA Targets" (2018). Honors Undergraduate Theses. 311. https://stars.library.ucf.edu/honorstheses/311 SPLIT DEOXYRIBOZYME PROBE FOR EFFICIENT DETECTION OF HIGHLY STRUCTURED RNA TARGETS By SHEILA SOLAREZ A thesis submitted in partial fulfillment of the requirements for the Honors in the Major Program in Biological Sciences in the College of Sciences and the Burnett Honors College at the University of Central Florida Orlando, Florida Spring Term, 2018 Thesis Chair: Yulia Gerasimova, PhD ABSTRACT Transfer RNAs (tRNAs) are known for their role as adaptors during translation of the genetic information and as regulators for gene expression; uncharged tRNAs regulate global gene expression in response to changes in amino acid pools in the cell. Aminoacylated tRNAs play a role in non-ribosomal peptide bond formation, post-translational protein labeling, modification of phospholipids in the cell membrane, and antibiotic biosynthesis. [1] tRNAs have a highly stable structure that can present a challenge for their detection using conventional techniques. -

Mrna Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection
International Journal of Molecular Sciences Review mRNA Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection 1, 1, 2 1,3, Shuqin Xu y, Kunpeng Yang y, Rose Li and Lu Zhang * 1 State Key Laboratory of Genetic Engineering, Institute of Genetics, School of Life Science, Fudan University, Shanghai 200438, China; [email protected] (S.X.); [email protected] (K.Y.) 2 M.B.B.S., School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China; [email protected] 3 Shanghai Engineering Research Center of Industrial Microorganisms, Shanghai 200438, China * Correspondence: [email protected]; Tel.: +86-13524278762 These authors contributed equally to this work. y Received: 30 July 2020; Accepted: 30 August 2020; Published: 9 September 2020 Abstract: Messenger ribonucleic acid (mRNA)-based drugs, notably mRNA vaccines, have been widely proven as a promising treatment strategy in immune therapeutics. The extraordinary advantages associated with mRNA vaccines, including their high efficacy, a relatively low severity of side effects, and low attainment costs, have enabled them to become prevalent in pre-clinical and clinical trials against various infectious diseases and cancers. Recent technological advancements have alleviated some issues that hinder mRNA vaccine development, such as low efficiency that exist in both gene translation and in vivo deliveries. mRNA immunogenicity can also be greatly adjusted as a result of upgraded technologies. In this review, we have summarized details regarding the optimization of mRNA vaccines, and the underlying biological mechanisms of this form of vaccines. Applications of mRNA vaccines in some infectious diseases and cancers are introduced. It also includes our prospections for mRNA vaccine applications in diseases caused by bacterial pathogens, such as tuberculosis. -
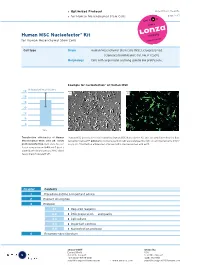
Optimized Protocol for Human Mesenchymal Stem Cells
› Optimized Protocol DPE-1001 Vs. 05-2005 › for Human Mesenchymal Stem Cells page 1 of 7 Human MSC Nucleofector® Kit for Human Mesenchymal Stem Cells Cell type Origin Human Mesenchymal Stem Cells (MSC), cryopreserved [Clonetics/BioWhittaker; Cat. No. PT-2501]. Morphology Cells with large nuclei and long spindle like protrusions.. Example for nucleofection® of Human MSC % transfection efficiency 60 A B 50 40 30 20 10 0 24 h Transfection efficiencies of Human Human MSC were nucleofected using the Human MSC Nucleofector Kit and a plasmid encoding the fluo- Mesenchymal Stem cells 24 hours rescent protein eGFP. 24 hours post-nucleofection cells were analyzed by light (A) and fluorescence micro- post nucleofection. Cells were nucleo- scopy (B). Transfection efficiencies of around 80% can be reached with eGFP. fected using program U-23 and 5 µg of a plasmid encoding the mouse MHC class I heavy chain molecule H-2Kk. Chapter Contents 1 Procedure outline & important advice 2 Product description 3 Protocol 3.1 › Required reagents 3.2 › DNA preparation and quality 3.3 › Cell culture 3.4 › Important controls 3.5 › Nucleofection protocol 4 Recommended literature 5 amaxa GmbH amaxa Inc. Europe/World USA Scientific Support Scientific Support +49 (0)221-99199-400 (240) 632-9110 [email protected] › www.amaxa.com [email protected] › Optimized Protocol DPE-1001 Vs. 05-2005 › for Human Mesenchymal Stem Cells page 2 of 7 1 Procedure outline & important advice Procedure outline Important advice 1. Preparation of cells. › Use MSCGM BulletKit (stored < 2 d at 4°C) (For details see 3.3.) › Passage interval: after reaching 70% confluency. -

Curevac Announces Financial Results for the Second Quarter and First Half of 2021 and Provides Business Update
CureVac Announces Financial Results for the Second Quarter and First Half of 2021 and Provides Business Update Continuation of corporate transformation into commercial-ready biopharma company o Management team strengthened with appointments of Chief Operating Officer Malte Greune and Chief Development Officer Klaus Edvardsen First-generation COVID-19 vaccine candidate, CVnCoV o Final analysis reported of pivotal Phase 2b/3 study conducted in ten countries in variant-dominated environment o Committed to seeking regulatory approval with EMA – submission of comprehensive clinical data packages within rolling submission ongoing Second-generation COVID-19 vaccine candidate, CV2CoV, jointly advanced with GSK o Preclinical study provides evidence for strongly improved immune responses with second-generation mRNA backbone compared to first-generation backbone during challenge study and against range of variants, including Delta and Lambda variant o Start of CV2CoV Phase 1 clinical trial anticipated in Q4 2021 Oncology Programs o Phase 1 trial extension of lead asset, CV8102, on track to fully recruit o Legacy program, BI 1361849, developed in partnership with Boehringer Ingelheim, based on early protamine formulation terminated; companies assessing options for further collaboration Financials: Cash position of €1.36 billion as of June 30, 2021 TÜBINGEN, Germany/ BOSTON, USA – August 16, 2021 – CureVac N.V. (Nasdaq: CVAC), a global biopharmaceutical company developing a new class of transformative medicines based on messenger ribonucleic acid (“mRNA”), today announced financial results for the second quarter and first half of 2021 and provided business update. “Large parts of the world are still under-vaccinated against SARS-CoV-2, making effective vaccines necessary to prevent further evolution of the virus and to avoid renewed restrictions on public life,” said Franz-Werner Haas, Chief Executive Officer of CureVac. -

J&J Provides Early Hope for Single-Dose Covid-19 Vaccine
January 14, 2021 J&J provides early hope for single-dose Covid-19 vaccine Madeleine Armstrong Pivotal data for JNJ-78436735 are imminent, with Novavax and Curevac not far behind. One advantage of Johnson & Johnson’s Covid-19 vaccine candidate is that it might provide protection after a single dose. Early neutralising antibody data suggest that this might indeed be the case – but, as ever, this needs to be confirmed with efficacy data. Luckily, that is coming very soon: results from the Ensemble trial, testing one dose of JNJ-78436735, are due in late January. Other projects from Novavax and Curevac are also set to yield pivotal data in the first quarter, so it might soon become clear whether more vaccines will be joining the currently authorised options. Selected upcoming readouts with Covid-19 vaccines Vaccine Company Trial name/details N Trial ID Timing name Ensemble, US ph3, single 45,000 NCT04505722 Data due Jan 2021 Johnson & JNJ- dose Johnson 78436735 Ensemble-2, US ph3, two 30,000 NCT04614948 Began Nov 2020 doses Data due early Q1 S Africa ph2b 4,400 NCT04533399 2021 NVX- Data due early Q1 Novavax UK ph3 15,000 NCT04583995 CoV2373 2021 Interim data due Q2 Prevent-19, US/Mexico ph3 30,000 NCT04611802 2021 Herald, Europe ph2/3 36,500 NCT04652102 Data due Q1 2021 CVnCoV Curevac Europe ph3, healthcare Primary completion 2,500 NCT04674189 workers Jun 2021 Primary completion Astrazeneca AZD1222* US ph3 30,000 NCT04516746 Mar 2021 *Authorised in the UK in Dec 2020. Source: EvaluatePharma & clinicaltrials.gov. The latest data came from a phase I/II trial of JNJ-78436735, and were published in the NEJM yesterday; these are updated results from a preprint released in September. -

Evaluation of Locked Nucleic Acid–Modified Small Interfering RNA in Vitro and in Vivo
833 Evaluation of locked nucleic acid–modified small interfering RNA in vitro and in vivo Olaf R. Mook, Frank Baas, Marit B. de Wissel, model. Therefore, LNA-modified siRNA should be pre- and Kees Fluiter ferred over unmodified siRNA. [Mol Cancer Ther 2007; 6(3):833–43] Department of Neurogenetics, Academic Medical Center, Amsterdam, the Netherlands Introduction RNA interference (RNAi) is a natural process that affects Abstract gene silencing in eukaryotic systems at transcriptional, RNA interference has become widely used as an experi- posttranscriptional, and/or translational levels (1). Small mental tool to study gene function. In addition, small interfering RNA (siRNA) molecules are the key intermedi- interfering RNA (siRNA) may have great potential for the ates in this process, which can potentially inhibit the treatment of diseases. Recently, it was shown that siRNA expression of any given target gene. siRNA molecules hold can be used to mediate gene silencing in mouse models. great promise as biological tools and as potential thera- Locally administered siRNAs entered the first clinical trials, peutic agents for targeted inhibition of disease-causing but strategies for successful systemic delivery of siRNA genes. are still under development. Challenges still exist about the To optimize the use of siRNA as a therapeutic therapy, stability, delivery, and therapeutic efficacy of siRNA. In several modes of delivery have been tested in vivo. the present study, we compare the efficacy of two Improved delivery in animals has been achieved by methods of systemic siRNA delivery and the effects of complexation with cationic liposomes (2), polyethyleni- siRNA modifications using locked nucleic acids (LNA) in a mine (3), and Arg-Gly-Asp–polyethylene glycol–polyethy- xenograft cancer model. -

Expanding the Genetic Code Lei Wang and Peter G
Reviews P. G. Schultz and L. Wang Protein Science Expanding the Genetic Code Lei Wang and Peter G. Schultz* Keywords: amino acids · genetic code · protein chemistry Angewandte Chemie 34 2005 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim DOI: 10.1002/anie.200460627 Angew. Chem. Int. Ed. 2005, 44,34–66 Angewandte Protein Science Chemie Although chemists can synthesize virtually any small organic molecule, our From the Contents ability to rationally manipulate the structures of proteins is quite limited, despite their involvement in virtually every life process. For most proteins, 1. Introduction 35 modifications are largely restricted to substitutions among the common 20 2. Chemical Approaches 35 amino acids. Herein we describe recent advances that make it possible to add new building blocks to the genetic codes of both prokaryotic and 3. In Vitro Biosynthetic eukaryotic organisms. Over 30 novel amino acids have been genetically Approaches to Protein encoded in response to unique triplet and quadruplet codons including Mutagenesis 39 fluorescent, photoreactive, and redox-active amino acids, glycosylated 4. In Vivo Protein amino acids, and amino acids with keto, azido, acetylenic, and heavy-atom- Mutagenesis 43 containing side chains. By removing the limitations imposed by the existing 20 amino acid code, it should be possible to generate proteins and perhaps 5. An Expanded Code 46 entire organisms with new or enhanced properties. 6. Outlook 61 1. Introduction The genetic codes of all known organisms specify the same functional roles to amino acid residues in proteins. Selectivity 20 amino acid building blocks. These building blocks contain a depends on the number and reactivity (dependent on both limited number of functional groups including carboxylic steric and electronic factors) of a particular amino acid side acids and amides, a thiol and thiol ether, alcohols, basic chain. -

Locked Nucleic Acid
lysi ana s & io B B io f m o e l d a Journal of Mishra and Mukhopadhyay, J Bioanal Biomed 2013, 5:2 i n c r i n u e DOI: 10.4172/1948-593X.1000e114 o J ISSN: 1948-593X Bioanalysis & Biomedicine Editorial OpenOpen Access Access Locked Nucleic Acid (LNA)-based Nucleic Acid Sensors Sourav Mishra and Rupa Mukhopadhyay* Department of Biological Chemistry, Indian Association for the Cultivation of Science, Jadavpur, Kolkata-700 032, India In recent times, much developments in the field of ‘nucleic acid may prevent interactions with the solid substrates, [15] and it can have sensors’, especially using alternative nucleic acid probes like peptide multiple water bridges that provide it with extra stability compared to nucleic acid (PNA) and locked nucleic acid (LNA), have taken place. DNA or RNA. It has been shown that both the highest Tm increase Although most of these are concerned with ‘solution phase’ studies, per LNA modification and the best mismatch discrimination are some reports have been made on ‘on-surface’ detection. The latter type achieved for short LNA sequences [16]. In addition to that, LNA of detection is particularly important to nurture, considering clinical phosphoramidites and their oligomers are commercially available, diagnostic applications using microarrays. In this article, we’ll briefly and LNA nucleotides can be mixed with those of the natural nucleic present the primary developments reported in the past two decades, acids for generating heterogeneous probe molecules. These properties along with possibilities for future developments, in case of the LNA- of LNA hold the promise that it can be a potentially better alternative based sensors. -

Malaria Parasites Both Repress Host CXCL10 and Use It As a Cue for Growth Acceleration
ARTICLE https://doi.org/10.1038/s41467-021-24997-7 OPEN Malaria parasites both repress host CXCL10 and use it as a cue for growth acceleration Yifat Ofir-Birin 1, Hila Ben Ami Pilo1, Abel Cruz Camacho1, Ariel Rudik1, Anna Rivkin1, Or-Yam Revach1, Netta Nir1, Tal Block Tamin1, Paula Abou Karam1, Edo Kiper1, Yoav Peleg2, Reinat Nevo1, Aryeh Solomon3, Tal Havkin-Solomon1, Alicia Rojas1, Ron Rotkopf 4, Ziv Porat 5, Dror Avni6,7, Eli Schwartz6,7, Thomas Zillinger 8, Gunther Hartmann 8, Antonella Di Pizio 9, Neils Ben Quashie 10,11, Rivka Dikstein1, Motti Gerlic12, Ana Claudia Torrecilhas 13, Carmit Levy14, Esther N. M. Nolte-‘t Hoen15, ✉ Andrew G. Bowie 16 & Neta Regev-Rudzki 1 1234567890():,; Pathogens are thought to use host molecular cues to control when to initiate life-cycle transitions, but these signals are mostly unknown, particularly for the parasitic disease malaria caused by Plasmodium falciparum. The chemokine CXCL10 is present at high levels in fatal cases of cerebral malaria patients, but is reduced in patients who survive and do not have complications. Here we show a Pf ‘decision-sensing-system’ controlled by CXCL10 concentration. High CXCL10 expression prompts P. falciparum to initiate a survival strategy via growth acceleration. Remarkably, P. falciparum inhibits CXCL10 synthesis in monocytes by disrupting the association of host ribosomes with CXCL10 transcripts. The underlying inhi- bition cascade involves RNA cargo delivery into monocytes that triggers RIG-I, which leads to HUR1 binding to an AU-rich domain of the CXCL10 3’UTR. These data indicate that when the parasite can no longer keep CXCL10 at low levels, it can exploit the chemokine as a cue to shift tactics and escape.