Role of Robot-Assisted Pelvic Surgery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Curative Pelvic Exenteration for Recurrent Cervical Carcinoma in the Era of Concurrent Chemotherapy and Radiation Therapy
Available online at www.sciencedirect.com ScienceDirect EJSO xx (2015) 1e11 www.ejso.com Review Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review H. Sardain a,b, V. Lavoue a,b,c,*, M. Redpath d, N. Bertheuil b,e, F. Foucher a,J.Lev^eque a,b,c a CHU de Rennes, Gynecology Department, Tertiary Surgery Center, Teaching Hospital of Rennes, Hopital^ Sud, 16, Bd de Bulgarie, 35000 Rennes, France b Universite de Rennes, Faculty of Medicine, 2 Henry Guilloux, 35000 Rennes, France c INSERM, ER440, Oncogenesis, Stress and Signaling (OSS), Rennes, France d McGill University, Department of Pathology, Jewish General Hospital, Cote^ Sainte Catherine, Montreal, QC, Canada e CHU de Rennes, Department of Plastic, Reconstructive and Aesthetic Surgery, Tertiary Surgery Center, Teaching Hospital of Rennes, Hopital^ Sud, 16, Bd de Bulgarie, 35000 Rennes, France Accepted 26 March 2015 Available online --- Abstract Objective: Pelvic exenteration requires complete resection of the tumor with negative margins to be considered a curative surgery. The pur- pose of this review is to assess the optimal preoperative evaluation and surgical approach in patients with recurrent cervical cancer to in- crease the chances of achieving a curative surgery with decreased morbidity and mortality in the era of concurrent chemoradiotherapy. Methods: Review of English publications pertaining to cervical cancer within the last 25 years were included using PubMed and Cochrane Library searches. Results: Modern imaging (MRI and PET-CT) does not accurately identify local extension of microscopic disease and is inadequate for pre- operative planning of extent of resection. -

Pelvic Exenteration for the Management of Pelvic Malignancies
Chapter 7 Pelvic Exenteration for the Management of Pelvic Malignancies Daniel Paramythiotis, Konstantinia Kofina and Antonios Michalopoulos Additional information is available at the end of the chapter http://dx.doi.org/10.5772/61083 Abstract Pelvic exenteration is a surgical procedure first described by Brunschwig in 1948 as a curative or palliative treatment for pelvic and perineal tumors. It is actually a radical operation, involving en bloc resection of pelvic organs, including reproductive structures, bladder, and rectosigmoid. In patients with recurrent cervical and vaginal malignancy, it is associated with a 5-year survival of more than 50%. In spite of advances in surgical management, consequences such as stomas, are still frequently unavoidable for radical tumor excision. Most candidates for this procedure have been diagnosed with recurrent cervical cancer that has previously been treated with surgery and radiation, or radiation alone. Complications of pelvic exenteration are more severe than those of standard resection of a colorectal carcinoma, so it is not commonly performed, including wound infection, wound dehiscence (also described as burst abdomen) the creation of fistulae (perineal-fecal, uretero-vaginal, between conduit and perineal wound), urinary tract infections, perineal hernias and intestinal obstruction. Patients need to be carefully selected and counseled about risks and long-term issues related to the surgery. A comprehensive evaluation is required in order to exclude unresectable or metastatic disease. Evolution of the technique through laparoscopy and minimally invasive surgery may result in a reduction of morbidity and mortality. Keywords: Pelvic exenteration, gynecologic cancer 1. Introduction Pelvic exenteration was first described by Brunschwig and his colleagues of New York’s Memorial Hospital in 1948 [1] and was initially performed as a palliative surgical intervention © 2015 The Author(s). -

Treating Cervical Cancer If You've Been Diagnosed with Cervical Cancer, Your Cancer Care Team Will Talk with You About Treatment Options
cancer.org | 1.800.227.2345 Treating Cervical Cancer If you've been diagnosed with cervical cancer, your cancer care team will talk with you about treatment options. In choosing your treatment plan, you and your cancer care team will also take into account your age, your overall health, and your personal preferences. How is cervical cancer treated? Common types of treatments for cervical cancer include: ● Surgery for Cervical Cancer ● Radiation Therapy for Cervical Cancer ● Chemotherapy for Cervical Cancer ● Targeted Therapy for Cervical Cancer ● Immunotherapy for Cervical Cancer Common treatment approaches Depending on the type and stage of your cancer, you may need more than one type of treatment. For the earliest stages of cervical cancer, either surgery or radiation combined with chemo may be used. For later stages, radiation combined with chemo is usually the main treatment. Chemo (by itself) is often used to treat advanced cervical cancer. ● Treatment Options for Cervical Cancer, by Stage Who treats cervical cancer? Doctors on your cancer treatment team may include: 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 ● A gynecologist: a doctor who treats diseases of the female reproductive system ● A gynecologic oncologist: a doctor who specializes in cancers of the female reproductive system who can perform surgery and prescribe chemotherapy and other medicines ● A radiation oncologist: a doctor who uses radiation to treat cancer ● A medical oncologist: a doctor who uses chemotherapy and other medicines to treat cancer Many other specialists may be involved in your care as well, including nurse practitioners, nurses, psychologists, social workers, rehabilitation specialists, and other health professionals. -

720Bfaa8bc7b73a78aff36ddef00
MOLECULAR AND CLINICAL ONCOLOGY 12: 237-243, 2020 A comparative study on the short‑term clinical efficacy of the modified laparoscopic uterine comminution technique and traditional methods XIAOJUN SHI1, LIBING SHI2 and SONGYING ZHANG2 1Department of Gynecology, The First Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang 314001; 2Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang 310016, P.R. China Received April 28, 2019; Accepted December 13, 2019 DOI: 10.3892/mco.2020.1982 Abstract. To assess the value of the modified laparoscopic diagnosis and treatment. It has thus become one of the most uterine comminution technique in laparoscopic uterine surgery, commonly used techniques in the field of gynecology. Uterine a total of 82 cases of laparoscopic myomectomy were divided fibroids are common benign tumors of the female genital into the traditional group and modified group, according to organs and the prevalence has been reported as high as 20 to a random number table. During the same period, 92 patients 40% (1,2). The widespread application of pulverizers allows who underwent laparoscopic hysterectomy were divided into for removal of uterine fibroids under laparoscopy, which better the conventional group and the modified group, according to a reflects the superiority of minimally invasive techniques. random number table. The patients in the conventional group However, preoperative diagnosis of uterine fibroids and and modified group who underwent laparoscopic uterine uterine sarcoma is very difficult. The incidence of uterine fibroid removal showed no significant differences in the fibroid sarcoma is approximately 0.03 to 1.00% (3). -
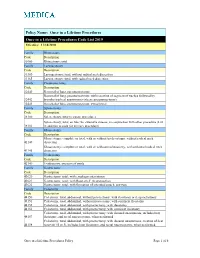
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Pelvic Exenteration As Ultimate Ratio for Gynecologic Cancers: Single‑Center Analyses of 37 Cases
Archives of Gynecology and Obstetrics (2019) 300:161–168 https://doi.org/10.1007/s00404-019-05154-4 GYNECOLOGIC ONCOLOGY Pelvic exenteration as ultimate ratio for gynecologic cancers: single‑center analyses of 37 cases N. de Gregorio1 · A. de Gregorio1 · F. Ebner2 · T. W. P. Friedl1 · J. Huober1 · R. Hefty3 · M. Wittau4 · W. Janni1 · P. Widschwendter1 Received: 5 February 2019 / Accepted: 5 April 2019 / Published online: 22 April 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Background Pelvic exenterations are a last resort procedure for advanced gynecologic malignancies with elevated risks in terms of patients’ morbidity. Methods This single-center analysis reports surgical details, outcome and survival of all patients treated with exenteration for non-ovarian gynecologic malignancies at our university hospital during a 13-year time period. We collected data regarding patients and tumor characteristics, surgical procedures, peri- and postoperative management, transfusions, complications, and analyzed the impact on survival outcomes. Results We identifed 37 patients between 2005 and 2013 with primary or relapsed cervical cancer (59.5%), vulvar cancer (24.3%) or endometrial cancer (16.2%). Median age was 60 years and most patients (73%) had squamous cell carcinomas. Median progression-free survival was 26.2 months and median overall survival was 49.9 months. The 5-year survival rates were 34.4% for progression-free survival and 46.4% for overall survival. There were no signifcant diferences in progres- sion-free survival and overall survival with regard to disease entity. Patients with tumor at the resection margins (R1) had a nearly signifcantly worse progression-free survival (median: 28.5 vs. -

Seer Program Code Manual
THE SEER PROGRAM CODE MANUAL Revised Edition June 1992 CANCER STATISTICS BRANCH SURVEILLANCE PROGRAM DIVISION OF CANCER PREVENTION AND CONTROL NATIONAL CANCER INSTITUTE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE NATIONAL INSTITUTES OF HEALTH Effective Date: Cases Diagnosed January 1, 1992 The SEER Program Code Manual Revised Edition June 1992 Editors Jack Cunningham Lynn Ries Benjamin Hankey Jennifer Seiffert Barbara Lyles Evelyn Shambaugh Constance Percy Valerie Van Holten Acknowledgements The editors wish to acknowledge the assistance of Terry Swenson, Maureen Troublefield, Diane Licitra, and Jerome Felix of Information Management Services, Inc., in the preparation of the SEER Program Code Manual. The editors also wish to acknowledge the assistance of Dr. John Berg in preparation of the section on multiple primary determination for lymphatic and hematopoietic diseases. TABLE OF CONTENTS PREFACE TO THE REVISED EDITION ....................................... vii COMPUTER RECORD FORMAT ............................................ 1 INTRODUCTION AND GENERAL INSTRUCTIONS .............................. 5 REFERENCES .......................................................... 37 SEER CODE SUMMARY .................................................. 39 I BASIC RECORD IDENTIFICATION ...................................... 57 1.01 SEER Participant ........................................... 58 1.02 Case Number .............................................. 59 1.03 Record Number ............................................ 60 -

Minimally Invasive Surgery for Uterine Fibroids
Ginekologia Polska 2020, vol. 91, no. 3, 149–157 Copyright © 2020 Via Medica REVIEW PAPER / GYNECOLOGY ISSN 0017–0011 DOI: 10.5603/GP.2020.0032 Minimally invasive surgery for uterine fibroids Yuehan Wang1 , Shitai Zhang1 , Chenyang Li2 , Bo Li1 , Ling Ouyang1 1Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China 2Shenyang Maternity and Child Health Hospital, Shenyang, China ABSTRACT The incidence of uterine fibroids, which comprise one of the most common female pelvic tumors, is almost 70–75% for women of reproductive age. With the development of surgical techniques and skills, more individuals prefer minimally invasive methods to treat uterine fibroids. There is no doubt that minimally invasive surgery has broad use for uterine fibroids. Since laparoscopic myomectomy was first performed in 1979, more methods have been used for uterine fibroids, such as laparoscopic hysterectomy, laparoscopic radiofrequency volumetric thermal ablation, and uterine artery emboliza- tion, and each has many variations. In this review, we compared these methods of minimally invasive surgery for uterine fibroids, analyzed their benefits and drawbacks, and discussed their future development. Key words: minimally invasive surgery; uterine fibroid; laparoscopic hysterectomy; laparoscopic myomectomy Ginekologia Polska 2020; 91, 3: 149–157 INTRODUCTION fibroids. They found that those two kinds of surgery did Uterine fibroids comprise one of the most common not have different recurrence risks, but that laparoscopic female pelvic tumors. When including the small, clinically myomectomy may be associated with less postoperative undetectable fibroids and microscopic fibroids, the incidence pain, lower postoperative fever, and shorter hospital stays is approximately 70–75% for those of reproductive age. -

Primary Pelvic Exenteration: Our Experience with 23 Patients from a Single Institution
EXPERIMENTAL AND THERAPEUTIC MEDICINE 22: 1060, 2021 Primary pelvic exenteration: Our experience with 23 patients from a single institution MIHAI GHEORGHE1, ALEXANDRA LAVINIA COZLEA1, SZILARD LEO KISS1, MIHAI STANCA1, MIHAI EMIL CĂPÎLNA1, NICOLAE BACALBAȘA2 and ANDREEA ANAMARIA MOLDOVAN3 1First Obstetrics and Gynecology Clinic, ‘George Emil Palade’ University of Medicine, Pharmacy, Science and Technology, 540136 Târgu Mureș; 2Department of Obstetrics and Gynecology, ‘Carol Davila’ University of Medicine and Pharmacy, 020022 Bucharest; 3Department of Infectious Diseases, Brașov County Emergency Hospital, 500326 Brașov, Romania Received May 24, 2021; Accepted June 23, 2021 DOI: 10.3892/etm.2021.10494 Abstract. This study was designed with an aim to share our Introduction experience of primary pelvic exenterations. The study included 23 patients with different types of pelvic cancer enrolled at a In patients with advanced primary or recurrent gyneco‑ single institution between November 2011 and July 2020. The logic (1), urologic or rectal cancers without metastatic disease, patient mean age was 55 years (range, 43‑72 years) and the extensive aggressive surgery such as pelvic exenteration may oncological indications included: Stage IVa cervical cancer be necessary for curative intent treatment (2). (11 cases, 48.9%), stage IVa endometrial cancer (1 case, 4.3%), Brunschwig was the first to describe pelvic exentera‑ stage IVa vaginal cancer (6 cases, 26%), stage IIIb bladder tion in the 1940s (3). Exenteration was initially considered cancer (3 cases, 13%), stage IIIc rectal cancer (1 case, 4.3%) as a palliative procedure for patients with extensive pelvic and undifferentiated pelvic sarcoma (1 case, 4.3%). Total, cancers, with an extremely high perioperative mortality rate anterior, and posterior pelvic exenterations were performed of 23%. -

Obstetrics and Gynecology Clinical Privilege List
Obstetrics and Gynecology Clinical Privilege List Description of Service Alberta Health Services (AHS) Medical Staff who are specialists in Obstetrics and Gynecology (or its associated subspecialties) and have privileges in the Department of Obstetrics and Gynecology provide safe, high quality care for obstetrical and gynecologic patients in AHS facilities across the province. The specialty encompasses medical, surgical, obstetrical and gynecologic knowledge and skills for the prevention, diagnosis and management of a broad range of conditions affecting women's gynecological and reproductive health. Working to provide a patient-focused, quality health system that is accessible and sustainable for all Albertans, the department also offers subspecialty care including gynecological oncology, reproductive endocrinology, maternal fetal medicine, urogynecology, and minimally invasive surgery.1 Obstetrics and Gynecology privileges may include admitting, evaluating, diagnosing, treating (medical and/or surgical management), to female patients of all ages presenting in any condition or stage of pregnancy or female patients presenting with illnesses, injuries, and disorders of the gynecological or genitourinary system including the ability to assess, stabilize, and determine the disposition of patients with emergent conditions consistent with medical staff policy regarding emergency and consultative call services. Providing consultation based on the designated position profile (clinical; education; research; service), and/or limited Medical Staff -

Gynaecology – Scope of Clinical Practice
Epworth HealthCare Gynaecology – Scope of Clinical Practice Gynaecology – Scope of Clinical Practice Clinical Institute: Women’s and Children’s Procedure Name Tier Adnexal surgery, including ovarian cystectomy, oophorectomy, salpingectomy, and Tier A conservative procedures for treatment of ectopic pregnancy Aspiration of breast masses Tier A Bladder biopsy and diathermy Tier A Cervical biopsy Tier A Colpocleisis Tier A Colpoplasty Tier A Colposcopy Tier A Consulting Tier A Cystoscopic injection of Botulinum Toxin Tier A Cystoscopy as part of gynaecological procedure Tier A D&C for abortion Tier A Delayed anal sphincter repair Tier A Diagnostic laparoscopy Tier A Endometrial ablation Tier A Exploratory laparotomy, for diagnosis and treatment of pelvic pain, pelvic mass, Tier A hemoperitoneum, endometriosis and adhesions Gynaecological sonography Tier A Hysterectomy, abdominal, vaginal, excluding laparoscopic Tier A Hysterosalpingography Tier A Hysteroscopic surgery Tier A Hysteroscopy Tier A I&D of bartholin cyst or perineal abscess Tier A I&D of pelvic abscess Tier A Incidental appendectomy Tier A Insertion and removal of IUCD Tier A Intrauterine myomectomy with power morcellation by hysteroscopic approach Tier A Laparoscopic surgery (excludes laparoscopic myomectomy with power morcellation) Tier A Laparotomy Tier A Marsupialisation of bartholin cysts Tier A Minor gynaecological surgical procedures (endometrial biopsy, dilation and curettage, Tier A treatment of Bartholin cyst and abscess) Myomectomy Tier A Operation for treatment of -

Case Report Uterine Fibroid Torsion During Pregnancy: a Case of Laparotomic Myomectomy at 18 Weeks’ Gestation with Systematic Review of the Literature
Hindawi Case Reports in Obstetrics and Gynecology Volume 2017, Article ID 4970802, 11 pages https://doi.org/10.1155/2017/4970802 Case Report Uterine Fibroid Torsion during Pregnancy: A Case of Laparotomic Myomectomy at 18 Weeks’ Gestation with Systematic Review of the Literature Annachiara Basso,1 Mariana Rita Catalano,1 Giuseppe Loverro,1 Serena Nocera,1 Edoardo Di Naro,1 Matteo Loverro,1 Mariateresa Natrella,2 and Salvatore Andrea Mastrolia1 1 Department of Obstetrics and Gynecology, Azienda Ospedaliera Universitaria Policlinico di Bari, School of Medicine, UniversitadegliStudidiBari“AldoMoro”,Bari,Italy` 2School of Nursing, Azienda Ospedaliera Universitaria Policlinico di Bari, School of Medicine, UniversitadegliStudidiBari“AldoMoro”,Bari,Italy` Correspondence should be addressed to Salvatore Andrea Mastrolia; [email protected] Received 15 January 2017; Revised 17 March 2017; Accepted 20 March 2017; Published 24 April 2017 Academic Editor: Maria Grazia Porpora Copyright © 2017 Annachiara Basso et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Uterine myomas are the most common benign growths affecting female reproductive system, occurring in 20–40% of women, whereas the incidence rate in pregnancy is estimated from 0.1 to 3.9%. The lower incidence in pregnancy is due to the association with infertility and low pregnancy rates and implantation rates after in vitro fertilization treatment. Uterine myomas, usually, are asymptomatic during pregnancy. However, occasionally, pedunculated fibroids torsion or other superimposed complications may cause acute abdominal pain. There are many controversies in performing myomectomy during cesarean section because of the risk of hemorrhage.