Alaska Shorebird Conservation Plan Version Ii
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Field Identification of Smaller Sandpipers Within the Genus <I
Field identification of smaller sandpipers within the genus C/dr/s Richard R. Veit and Lars Jonsson Paintings and line drawings by Lars Jonsson INTRODUCTION the hand, we recommend that the reader threeNearctic species, the Semipalmated refer to the speciesaccounts of Prateret Sandpiper (C. pusilia), the Western HESMALL Calidris sandpipers, affec- al. (1977) or Cramp and Simmons Sandpiper(C. mauri) andthe LeastSand- tionatelyreferred to as "peeps" in (1983). Our conclusionsin this paperare piper (C. minutilla), and four Palearctic North America, and as "stints" in Britain, basedupon our own extensivefield expe- species,the primarilywestern Little Stint haveprovided notoriously thorny identi- rience,which, betweenus, includesfirst- (C. minuta), the easternRufous-necked ficationproblems for many years. The hand familiarity with all sevenspecies. Stint (C. ruficollis), the eastern Long- first comprehensiveefforts to elucidate We also examined specimensin the toed Stint (C. subminuta)and the wide- thepicture were two paperspublished in AmericanMuseum of Natural History, spread Temminck's Stint (C. tem- Brtttsh Birds (Wallace 1974, 1979) in Museumof ComparativeZoology, Los minckii).Four of thesespecies, pusilla, whichthe problem was approached from Angeles County Museum, San Diego mauri, minuta and ruficollis, breed on the Britishperspective of distinguishing Natural History Museum, Louisiana arctictundra and are found during migra- vagrant Nearctic or eastern Palearctic State UniversityMuseum of Zoology, tion in flocksof up to thousandsof -

To View the Apr/May Issue of the Sandpiper (Pdf)
The andpiper APRIL/MAY 2018 Redwood Region Audubon Society www.rras.org S APRIL/MAY FIELD TRIPS Every Saturday: Arcata Marsh and Wildlife Sanctuary. Sunday, April 8: Humboldt Bay National Wildlife carpooling available. Walks generally run 2-3 hours. All These are our famous, rain-or-shine, docent-led fi eld trips at Refuge. This is a wonderful 2-to 3-hour trip for people ages, abilities and interest levels welcome! For more the Marsh. Bring your binocular(s) and have a great morning wanting to learn the birds of the Humboldt Bay area. It information, please contact Melissa Dougherty at 530-859- birding! Meet in the parking lot at the end of South I Street takes a leisurely pace with emphasis on enjoying the birds! 1874 or email [email protected]. (Klopp Lake) in Arcata at 8:30 a.m. Trips end around 11 a.m. Beginners are more than welcome. Meet at the Refuge Walks led by: Cédric Duhalde (Apr 7); Cindy Moyer (Apr Visitor Center at 9 a.m. Call Jude Power (707-822- 3613) Saturday, April 14: Shorebird Workshop, Part 14); Michael Morris (Apr 21); Christine Keil (Apr 28). If you for more information. III at Del Norte Pier. Meet at 10 a.m. to watch the are interested in leading a Marsh walk, please contact Ken rising tide at the foot of W. Del Norte St. bring in waves Burton at [email protected]. Sunday, April 8: Shorebird Workshop, Part II of godwits, willets, turnstones, and curlews. Tide will turn at South Spit. First we’ll look for beach-loving around noon; we hope to see a good show by then. -

Arcata Marsh & Wildlife Sanctuary Bird Checklist
Arcata Marsh & Wildlife Sanctuary Bird Checklist Arcata, Humboldt County, California (Updated Fall 2014) The following list of 327 species was updated by Rob Fowler and David Fix in 2014 from the list they compiled in 2009. Data came from sightings entered in eBird; Stanley Harris's Northwest California Bird (2005, 1996, 1991); historical records in North American Birds magazine and its supporting unpublished Humboldt County summaries; the 2006 edition Arcata Marsh bird checklist (Elias Elias); the 1995 edition Arcata Marsh bird checklist (Kristina Van Wert); and personal communications with many birders. Formatting by Camden Bruner. Call the Northwest California Bird Alert at (707) 822-5666 to report or hear reports of rare birds! Abbreviations: A - Abundant; occurs in large numbers C - Common; likely to be found U - Uncommon; occurs in small numbers, found with seearching R - Rare; expected in very small numbers, not likely to be found Ca - Casual; several records, possibly may occur regularly Ac - Accidental; 1-3 records, not reasonably expected to occur Sp - Spring (Marsh - May) S - Summer (June to mid-July) F - Fall (mid-July through November) W - Winter (December through February) Here Waterfowl: Breeds Spring Summer Fall Winter _____ Greater White-fronted Goose R R R _____ Emperor Goose Ac _____ Snow Goose Ca Ca Ca _____ Ross's Goose Ca Ca Ca _____ Brant U Ac U R _____ Cackling Goose A U C _____ Canada Goose C C C C yes _____ Tundra Swan Ca Ca _____ Wood Duck U U U U yes _____ Gadwall C C C C yes _____ Eurasian Wigeon R U R _____ -

Species Account
SPOTTED SANDPIPER Actitis macularius non-breeding visitor, vagrant monotypic Spotted Sandpipers breed across n. N America and winter as far south as c. S America (AOU 1998). The status of this species in the Pacific and the Hawaiian Islands is confused by its similarity to Common Sandpiper, a Eurasian counterpart (Dement'ev and Gladkov 1951c, Cramp and Simmons 1983), that has reached the Hawaiian Islands on at least two occasion and possibly others (David 1991). Records of this pair, unidentified to species, have been reported throughout the Pacific (E 41:115, Clapp 1968a, Pyle and Engbring 1985, Pratt et al. 1987) while confirmed Spotted Sandpipers have been recorded from Clipperton, the Marshall, Johnston, and the Hawaiian Is (Amerson and Shelton 1976, Howell et al. 1993, AOU 1998). David (1991) analyzed records of the two species of Actitis sandpipers in the Southeastern Hawaiian Islands and concluded that, between 1975 and 1989, 6 of 12 birds (1983-1989) could be confirmed as Spotted Sandpipers based on descriptions and photographs while the remaining six (1975-1983) could not be identified. Prior to this, Pyle (1977) listed only the species pair (Spotted/Common Sandpiper) for the Hawaiian Islands. Since this analysis and through the 2000s there have been 25 additional records of Actitis, 18 of which we consider confirmed Spotted Sandpipers while 7 did not include enough descriptive notes to separate them from Common Sandpiper. Because 24 of 37 records in the Southeastern Islands have been confirmed as Spotted Sandpipers and only one has been confirmed as a Common Sandpiper, we assume that the following summary of Actitis sandpipers reflects the status of Spotted Sandpiper, the more expected species in the Southeastern Islands. -

Hudsonian Godwit (Limosa Haemastica) at Orielton Lagoon, Tasmania, 09/03/2018
Hudsonian Godwit (Limosa haemastica) at Orielton Lagoon, Tasmania, 09/03/2018 Peter Vaughan and Andrea Magnusson E: T: Introduction: This submission pertains to the observation and identification of a single Hudsonian Godwit (Limosa haemastica) at Orielton Lagoon, Tasmania, on the afternoon of the 9th of March 2018. The bird was observed in the company of 50 Bar-tailed Godwits (Limosa lapponica) and a single Black-tailed Godwit (Limosa limosa), and both comparative and diagnostic features were used to differentiate it from these species. Owing to the recognised difficulties in differentiating similar species of wading birds, this submission makes a detailed analysis of the features used to identify the godwit in question. If accepted, this sighting would represent the eighth confirmed record of this species in Australia, and the second record of this species in Tasmania. Sighting and Circumstances: The godwit in question was observed on the western side of Orielton Lagoon in Tasmania, at approximately 543440 E 5262330 N, GDA 94, 55G. This location is comprised of open tidal mudflats abutted by Sarcicornia sp. saltmarsh, and is a well- documented roosting and feeding site for Bar-tailed Godwits and several other species of wading bird. This has included, for the past three migration seasons, a single Black-tailed Godwit (extralimital at this location) associating with the Bar-tailed Godwit flock. The Hudsonian Godwit was observed for approximately 37 minutes, between approximately 16:12 and 16:49, although the mixed flock of Bar-tailed Godwits and Black-tailed Godwits was observed for 55 minutes prior to this time. The weather during observation was clear skies (1/8 cloud cover), with a light north- easterly breeze and relatively warm temperatures (wind speed nor exact temperature recorded). -

<I>Actitis Hypoleucos</I>
Partial primary moult in first-spring/summer Common Sandpipers Actitis hypoleucos M. NICOLL 1 & P. KEMP 2 •c/o DundeeMuseum, Dundee, Tayside, UK 243 LochinverCrescent, Dundee, Tayside, UK Citation: Nicoll, M. & Kemp, P. 1983. Partial primary moult in first-spring/summer Common Sandpipers Actitis hypoleucos. Wader Study Group Bull. 37: 37-38. This note is intended to draw the attention of wader catch- and the old inner feathersare often retained (Pearson 1974). ers to the needfor carefulexamination of the primariesof Similarly, in Zimbabwe, first-year Common Sandpipers CommonSandpipers Actiris hypoleucos,and other waders, replacethe outerfive to sevenprimaries between December for partial primarywing moult. This is thoughtto be a diag- andApril (Tree 1974). It thusseems normal for first-spring/ nosticfeature of wadersin their first spring and summer summerCommon Sandpipers wintering in eastand southern (Tree 1974). Africa to show a contrast between new outer and old inner While membersof the Tay Ringing Group were mist- primaries.There is no informationfor birdswintering further nettingin Angus,Scotland, during early May 1980,a Com- north.However, there may be differencesin moult strategy mon Sandpiperdied accidentally.This bird was examined betweenwintering areas,since 3 of 23 juvenile Common and measured, noted as an adult, and then stored frozen un- Sandpiperscaught during autumn in Morocco had well- til it was skinned,'sexed', andthe gut contentsremoved for advancedprimary moult (Pienkowski et al. 1976). These analysis.Only duringskinning did we noticethat the outer birdswere moultingnormally, and so may have completed primarieswere fresh and unworn in comparisonto the faded a full primary moult during their first winter (M.W. Pien- and abradedinner primaries.The moult on both wingswas kowski, pers.comm.). -

4/15/2015 - Rk
4/15/2015 - rk 7. 8. 9. 10. 11. 12. 13. 4/15/2015 - rk PUBLIC ARTS COMMITTEE UNAPPROVED REGULAR MEETING FEBRUARY 12, 2015 Session 15-01 a Regular Meeting of the Public Arts Committee was called to order on February 12, 2015 at 5:00 pm by Chair Michele Miller at the Homer City Hall Upstairs Conference Room located at 491 E. Pioneer Avenue, Homer, Alaska. PRESENT: COMMITTEE MEMBERS MILLER, HOLLOWELL AND PETERSEN ABSENT: COMMITTEE MEMBERS GRONING-PERSON AND APLIN (EXCUSED) STAFF: RENEE KRAUSE, CMC, DEPUTY CITY CLERK I The Committee met in a worksession from 4:00 p.m. until 4:45 p.m. Discussion on applications for a grant this year and the need to determine a suitable project and the draft Request for Proposal for a Consultant/Contractor to Inventory the Municipal Art Collection APPROVAL OF THE AGENDA Chair Miller requested a motion to approve the agenda HOLLOWELL/PETERSEN – MOVED TO APPROVE. There was no discussion. The agenda was approved by consensus of the committee. APPROVAL OF THE MINUTES (Minutes are approved during regular or special meetings only) A. Meeting Minutes for regular meeting of November 13, 2014. Chair Miller inquired if there was any issue with the minutes. Hearing none she requested a motion to approve the minutes as presented. PETERSEN/HOLLOWELL – MOVED TO APPROVE THE MINUTES. There was a brief discussion on seeing a possible misspelling but it was not immediately located within the document. Staff will review and correct. The minutes were approved by consensus of the Committee. PUBLIC COMMENTS ON ITEMS ALREADY ON THE AGENDA There was no public present. -

Green Sandpiper Uttering Call of Wood Sandpiper.—With Reference to J
NOTES Gannets robbing gulls.—On 5th August 1957, I spent the day at sea in Dingle Bay, Co. Kerry, in the trawler "Ros Brin". As soon as trawling operations began, we were accompanied by a few Storm Petrels (Hydrobates pelagicus), Fulmars (Fulmarus glacialis), Great Black-backed Gulls (Larus marinus), Lesser Black-backed Gulls (L. fuscus), and large numbers of Herring Gulls (L. argentatus). During the gutting periods, and especially when any small waste fish were thrown over-board, six to eight Gannets (Sula bassana), would join in the feast. Any fish that sank too deep for the gulls were immediately taken by the Gannets by the normal manner of diving, but on numerous occasions when a gull sitting on the surface had a fish it could not swallow quickly—i.e. small plaice, dabs and other flat fish—one or other of the Gannets would plunge in a shallow-angled frontal dive at the gull, entering the water about two yards away and snatching the fish from the gull's bill on breaking the surface. The fish was swallowed on the surface. This was repeated many times, and the Gannets also frequently fluttered along the water and snatched fish from the bills of other gulls which they passed. On several occasions, too, a Gannet was seen to lunge into the air after a retreating gull that was attempting to rise from the water with a fish; the Gannet "treading water" with wings half-open and neck fully extended upwards in its endeavour to snap the fish from the slowly rising and heavily gorged' gull. -

Tringarefs V1.3.Pdf
Introduction I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2016 & 2017 to: [email protected]. Please note that this and other Reference Lists I have compiled are not exhaustive and best employed in conjunction with other reference sources. Grateful thanks to Graham Clarke (http://grahamsphoto.blogspot.com/) and Tom Shevlin (www.wildlifesnaps.com) for the cover images. All images © the photographers. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds). 2016. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 6.1 accessed February 2016]). Version Version 1.3 (March 2016). Cover Main image: Spotted Redshank. Albufera, Mallorca. 13th April 2011. Picture by Graham Clarke. Vignette: Solitary Sandpiper. Central Bog, Cape Clear Island, Co. Cork, Ireland. 29th August 2008. Picture by Tom Shevlin. Species Page No. Greater Yellowlegs [Tringa melanoleuca] 14 Green Sandpiper [Tringa ochropus] 16 Greenshank [Tringa nebularia] 11 Grey-tailed Tattler [Tringa brevipes] 20 Lesser Yellowlegs [Tringa flavipes] 15 Marsh Sandpiper [Tringa stagnatilis] 10 Nordmann's Greenshank [Tringa guttifer] 13 Redshank [Tringa totanus] 7 Solitary Sandpiper [Tringa solitaria] 17 Spotted Redshank [Tringa erythropus] 5 Wandering Tattler [Tringa incana] 21 Willet [Tringa semipalmata] 22 Wood Sandpiper [Tringa glareola] 18 1 Relevant Publications Bahr, N. 2011. The Bird Species / Die Vogelarten: systematics of the bird species and subspecies of the world. Volume 1: Charadriiformes. Media Nutur, Minden. Balmer, D. et al 2013. Bird Atlas 2001-11: The breeding and wintering birds of Britain and Ireland. -

Biogeographical Profiles of Shorebird Migration in Midcontinental North America
U.S. Geological Survey Biological Resources Division Technical Report Series Information and Biological Science Reports ISSN 1081-292X Technology Reports ISSN 1081-2911 Papers published in this series record the significant find These reports are intended for the publication of book ings resulting from USGS/BRD-sponsored and cospon length-monographs; synthesis documents; compilations sored research programs. They may include extensive data of conference and workshop papers; important planning or theoretical analyses. These papers are the in-house coun and reference materials such as strategic plans, standard terpart to peer-reviewed journal articles, but with less strin operating procedures, protocols, handbooks, and manu gent restrictions on length, tables, or raw data, for example. als; and data compilations such as tables and bibliogra We encourage authors to publish their fmdings in the most phies. Papers in this series are held to the same peer-review appropriate journal possible. However, the Biological Sci and high quality standards as their journal counterparts. ence Reports represent an outlet in which BRD authors may publish papers that are difficult to publish elsewhere due to the formatting and length restrictions of journals. At the same time, papers in this series are held to the same peer-review and high quality standards as their journal counterparts. To purchase this report, contact the National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161 (call toll free 1-800-553-684 7), or the Defense Technical Infonnation Center, 8725 Kingman Rd., Suite 0944, Fort Belvoir, VA 22060-6218. Biogeographical files o Shorebird Migration · Midcontinental Biological Science USGS/BRD/BSR--2000-0003 December 1 By Susan K. -

NSF 03-021, Arctic Research in the United States
This document has been archived. Home is Where the Habitat is An Ecosystem Foundation for Wildlife Distribution and Behavior This article was prepared The lands and near-shore waters of Alaska remaining from recent geomorphic activities such by Page Spencer, stretch from 48° to 68° north latitude and from 130° as glaciers, floods, and volcanic eruptions.* National Park Service, west to 175° east longitude. The immense size of Ecosystems in Alaska are spread out along Anchorage, Alaska; Alaska is frequently portrayed through its super- three major bioclimatic gradients, represented by Gregory Nowacki, USDA Forest Service; Michael imposition on the continental U.S., stretching from the factors of climate (temperature and precipita- Fleming, U.S. Geological Georgia to California and from Minnesota to tion), vegetation (forested to non-forested), and Survey; Terry Brock, Texas. Within Alaska’s broad geographic extent disturbance regime. When the 32 ecoregions are USDA Forest Service there are widely diverse ecosystems, including arrayed along these gradients, eight large group- (retired); and Torre Arctic deserts, rainforests, boreal forests, alpine ings, or ecological divisions, emerge. In this paper Jorgenson, ABR, Inc. tundra, and impenetrable shrub thickets. This land we describe the eight ecological divisions, with is shaped by storms and waves driven across 8000 details from their component ecoregions and rep- miles of the Pacific Ocean, by huge river systems, resentative photos. by wildfire and permafrost, by volcanoes in the Ecosystem structures and environmental Ring of Fire where the Pacific plate dives beneath processes largely dictate the distribution and the North American plate, by frequent earth- behavior of wildlife species. -
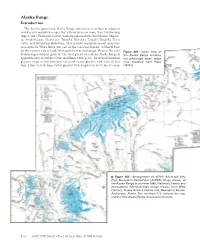
Alaska Range
Alaska Range Introduction The heavily glacierized Alaska Range consists of a number of adjacent and discrete mountain ranges that extend in an arc more than 750 km long (figs. 1, 381). From east to west, named ranges include the Nutzotin, Mentas- ta, Amphitheater, Clearwater, Tokosha, Kichatna, Teocalli, Tordrillo, Terra Cotta, and Revelation Mountains. This arcuate mountain massif spans the area from the White River, just east of the Canadian Border, to Merrill Pass on the western side of Cook Inlet southwest of Anchorage. Many of the indi- Figure 381.—Index map of vidual ranges support glaciers. The total glacier area of the Alaska Range is the Alaska Range showing 2 approximately 13,900 km (Post and Meier, 1980, p. 45). Its several thousand the glacierized areas. Index glaciers range in size from tiny unnamed cirque glaciers with areas of less map modified from Field than 1 km2 to very large valley glaciers with lengths up to 76 km (Denton (1975a). Figure 382.—Enlargement of NOAA Advanced Very High Resolution Radiometer (AVHRR) image mosaic of the Alaska Range in summer 1995. National Oceanic and Atmospheric Administration image mosaic from Mike Fleming, Alaska Science Center, U.S. Geological Survey, Anchorage, Alaska. The numbers 1–5 indicate the seg- ments of the Alaska Range discussed in the text. K406 SATELLITE IMAGE ATLAS OF GLACIERS OF THE WORLD and Field, 1975a, p. 575) and areas of greater than 500 km2. Alaska Range glaciers extend in elevation from above 6,000 m, near the summit of Mount McKinley, to slightly more than 100 m above sea level at Capps and Triumvi- rate Glaciers in the southwestern part of the range.