A Picture Is Worth a Thousand Words
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Age-Related Features and Pathology of Blood in Children
MINISTRY OF PUBLIC HEALTH OF UKRAINE HIGHER STATE EDUCATIONAL ESTABLISHMENT OF UKRAINE «UKRAINIAN MEDICAL STOMATOLOGICAL ACADEMY» V.I. POKHYLKO, S.M. TSVIRENKO, YU.V. LYSANETS AGE-RELATED FEATURES AND PATHOLOGY OF BLOOD IN CHILDREN MANUAL FOR STUDENTS OF HIGHER MEDICAL EDUCATIONAL INSTITUTIONS OF THE III-IV ACCREDITATION LEVELS Poltava 2017 МІНІСТЕРСТВО ОХОРОНИ ЗДОРОВ’Я УКРАЇНИ ВИЩИЙ ДЕРЖАВНИЙ НАВЧАЛЬНИЙ ЗАКЛАД УКРАЇНИ «УКРАЇНСЬКА МЕДИЧНА СТОМАТОЛОГІЧНА АКАДЕМІЯ» ПОХИЛЬКО В.І., ЦВІРЕНКО С.М., ЛИСАНЕЦЬ Ю.В. ВІКОВІ ОСОБЛИВОСТІ ТА ПАТОЛОГІЯ КРОВІ У ДІТЕЙ НАВЧАЛЬНИЙ ПОСІБНИК ДЛЯ СТУДЕНТІВ ВИЩИХ МЕДИЧНИХ НАВЧАЛЬНИХ ЗАКЛАДІВ III-IV РІВНІВ АКРЕДИТАЦІЇ Полтава 2017 2 UDC: 616+616.15]-053.2(075.8) ВВС: 57.33я73 The manual highlights the issues of embryogenesis, age-related features, semiotics of lesion, examination methods and diseases of hemic system in children. The manual is intended for students of higher educational institutions of III-IV accreditation levels, and can be used by medical interns and primary care doctors. Authors: Doctor of Medical Sciences, Professor of the Department of Pediatrics No.1 with Propedeutics and Neonatology V.I. Pokhylko Candidate of Medical Sciences, Acting Head of the Department of Pediatrics No.1 with Propedeutics and Neonatology S.M. Tsvirenko Candidate of Philological Sciences, Senior Lecturer of the Department of Foreign Languages with Latin and Medical Terminology Yu.V. Lysanets Reviewers: O.S. Yablon’ ― Doctor of Medical Sciences, Professor, Head of the Department of Pediatrics No.1, Vinnytsya National M.I. Pirogov Memorial Medical University of Ministry of Public Health of Ukraine. M.O. Honchar ― Doctor of Medical Sciences, Professor, Head of the Department of Pediatrics and Neonatology No.1, Kharkiv National Medical University. -
![Anormal Rbc in Peripheral Blood. [Repaired].Pdf](https://docslib.b-cdn.net/cover/4277/anormal-rbc-in-peripheral-blood-repaired-pdf-544277.webp)
Anormal Rbc in Peripheral Blood. [Repaired].Pdf
1. Acanthocyte 2. Burr-cell 3. Microcyte 1. Basophilic Normoblast 2. Polychromatic Normoblast 3. Pycnotic Normoblast 4. Plasmocyte 5. Eosinophil 6. Promyelocyte 1. Macrocyte 2. Elliptocyte 1. Microcyte 2. Normocyte 1. Polychromatic Erythrocyte 2. Acanthocyte 3. Elliptocyte 1. Polychromatic Normoblast 2. Pycnotic Normoblast 3. Neutrophil Myelocyte 4. Neutrophil Metamyelocyte 1. Schistocyte 2. Microcyte BASOPHILIC ( EARLY ) NORMOBLASTS Basophilic Erythroblast Basophilic Stippling, Blood smear, May-Giemsa stain, (×1000) CABOT'S RINGS Drepanocyte Elliptocyte Erythroblast ERYTHROBLAST in the blood Howell-jolly body Hypo chromic LACRYMOCYTES Leptocyte Malaria, Blood smear, May-Giemsa stain, ×1000 MICROCYTES Orthochromatic erythroblast Pappen heimer Bodies & 1. Schistocyte 2. Elliptocyte 3. Acanthocyte POIKILOCYTOSIS Polychromatic Erythroblast Pro Erytroblast Proerythroblasts Reticulocyte Rouleaux SICKLE CELLS Sickle cell Spherocyte Spherocyte Spherocyte SPHEROCYTES STOMATOCYTES Target Cells Tear Drop Cell, Blood smear, May-Giemsa stain, x1000 Anulocyte 1. Burr-cell 2. Elliptocyte 1. Macrocyte 2. Microcyte 3. Elliptocyte 4. Schistocyte 1. Ovalocyte 2. Lacrymocyte 3. Target cell 1. Polychromatic Erythrocyte 2. Basophilic Stippling 1. Proerythroblast 2. Basophilic Erythroblast 3. Intermediate Erythroblast 4. Late Erythroblast 5. Monocyte 6. Lymphocyte 1. Target-cell 2. Elliptocyte 3. Acanthocyte 4. Stomatocyte 5. Schistocyte 6. Polychromatophilic erythrocyte. 1.Pro erythroblast 2.Basophilic normoblast 3.Polychromatic normoblast 4.Pycnotic normoblast -

Red Blood Disorders Anemia Med.Pdf 14.44MB
Lviv National Medical University Department of pathological physiology PATHOLOGY OF RED BLOOD PhD. Sementsiv N.G. In norm The number of erythrocytes: in female - 3,9-4,7·1012/l in male - 4,5-5,0·1012/l Hemoglobin in female - 120-140g/l in male - 140-160g/l Color index(CI) - 0,85-1,15 Globular value = 3 x Hb / the first 3 figures of erythrocytes. Reticulocytes - 0.5-2%, 0,5-2%0 changes in total blood volume normovolemia hypovolemia hypervolemia simple (Ht - norm), polycitemia (Ht > 0,48), olygocytemia (Ht < 0,36). A) Norm B) acut anaemia б) acute hemorrhage г) hydremia Pathological forms of erythrocytes regenerative degenerative cell pathologic regeneration Regenerative forms reticulocytes залежно від зрілості розрізняють: (Зернисті) Stippling (Сітчасті) Mesh Norm in a blood reticulocytes - 0,2–2,0%. Regenerative forms Basophiles substantial erythrocytes - cytoplasm remains basophilic normo blast. Polychromatophil erythrocytes (polychromasia, polychromatophilia ) – erythrocytes with basophiles substantial ( blue cells) indicates increased RBC production by the marrow Qualitative (degenerative) changes of red blood cell - poikilocytosis - different shape of erythrocytes; - anisocytosis - different size of erythrocytes; - anisochromia - different saturation of red blood cells by hemoglobin Degenerative forms Anisocytosis present in a blood different forms erythrocytes » normocyte (7,01–8,0 мкм) » microcyte(6,9–5,7 мкм) » macrocyte(8,1–9,35 мкм) » megalocyte (10–15 мкм) Degenerative forms Poikilocytosis present in a blood pictures erythrocytes different forms : elongate form , oval, ellipsoid and os. ОVAlOCYTE( ELLIPTOCYTE) – 5% all blood. Pathological cells regenerate Megaloblast mehaloblastyc cell type hematopoiesis Megaloblast oval cells in the diameter of 1,5- 2,0 times larger than normal erythrocytes is the final stage mehaloblastyc hematopoiesis. -

Cambridge University Press 978-0-521-51426-2 — Anemia with Online Resource Edited by Edward J
Cambridge University Press 978-0-521-51426-2 — Anemia with Online Resource Edited by Edward J. Benz, Jr. , Nancy Berliner , Fred J. Schiffman Index More Information Index aAA. See acquired aplastic clinical significance of, 187 through RBC transfusion, anemia of inflammation, 169 anemia dementia, 187 175 anemia of prematurity, 34 abdomen, physical examination epidemiology of, 185–6 with rhEPO, 172, 174–7 anemias. See also clinical of, 32 inflammation and, 186–7 pathogenesis of, 172–3 approaches; specific ABO incompatibility, 36 hepcidin levels, 186 pathophysiology, 198 anemias HSCT and, 181 leptin protein, 186 therapy-related toxicity causes of, xi, 1 acanthocytes, 27 MIF, 186 grading, 172 through blood loss, 24 ACD. See anemia of chronic TNF-α, 186 transfusion, 175 classification of, 24 inflammation vitamin D deficiency, 186–7 anemia of chronic inflammation through MCV, 24 ACI. See anemia of chronic management strategies, 187–8 (ACI) clinical definitions of, xi, 23 inflammation prevalence of, 185 cancer and, 172 comorbidities with, xi acquired aplastic anemia (aAA), aHUS. See atypical hemolytic case study for, 153–4 defined, 172, 185 128–32. See also uremic syndrome clinical presentation, 152–3, diagnostic approach, 23–4 paroxysmal nocturnal AIHA. See autoimmune 194 through bone marrow hemoglobinuria hemolytic anemia critical illness with, 151 aspirate specimens, 24 bone marrow with, 128 alcohol use, non-megaloblastic cytokines, 196 through peripheral blood clinical presentation of, 129 macrocytic anemias diagnostic evaluation, 153, smears, 24–5, 29 clonal hematopoiesis and, 129 from, 63 194–5 future approaches to, 230–3 diagnostic evaluation of, alemtuzumab, 134 epidemiology, 150–1 hemoglobin deficiency in, xi 129–30 ALI. -

10 11 Cyto Slides 81-85
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TESTING PROGRAM Glass Slide Critique ~ November 2010 Slide 081 Diagnosis: MDS to AML 9 WBC 51.0 x 10 /L 12 Available data: RBC 3.39 x 10 /L 72 year-old female Hemoglobin 9.6 g/dL Hematocrit 29.1 % MCV 86.0 fL Platelet count 16 x 109 /L The significant finding in this case of Acute Myelogenous Leukemia (AML) was the presence of many blast forms. The participant median for blasts, all types was 88. The blast cells in this case (Image 081) are large, irregular in shape and contain large prominent nucleoli. It is difficult to identify a blast cell as a myeloblast without the presence of an Auer rod in the cytoplasm. Auer rods were reported by three participants. Two systems are used to classify AML into subtypes, the French- American-British (FAB) and the World Health Organization (WHO). Most are familiar with the FAB classification. The WHO classification system takes into consideration prognostic factors in classifying AML. These factors include cytogenetic test results, patient’s age, white blood cell count, pre-existing blood disorders and a history of treatment with chemotherapy and/or radiation therapy for a prior cancer. The platelet count in this case was 16,000. Reduced number of platelets was correctly reported by 346 (94%) of participants. Approximately eight percent of participants commented that the red blood cells in this case were difficult to evaluate due to the presence of a bluish hue around the red blood cells. Comments received included, “On slide 081 the morphology was difficult to evaluate since there was a large amount of protein surrounding RBC’s”, “Slide 081 unable to distinguish red cell morphology due to protein” and “Unable to adequately assess morphology on slide 081 due to poor stain”. -

Pathological Physiology
PATHOLOGICAL PHYSIOLOGY PRACTICAL PART Student _____________________________ Group number ________________________ Teacher _____________________________ Minsk BSMU 2015 МИНИСТЕРСТВО ЗДРАВООХРАНЕНИЯ РЕСПУБЛИКИ БЕЛАРУСЬ БЕЛОРУССКИЙ ГОСУДАРСТВЕННЫЙ МЕДИЦИНСКИЙ УНИВЕРСИТЕТ КАФЕДРА ПАТОЛОГИЧЕСКОЙ ФИЗИОЛОГИИ ПАТОЛОГИЧЕСКАЯ ФИЗИОЛОГИЯ PATHOLOGICAL PHYSIOLOGY Практикум 4-е издание Минск БГМУ 2015 2 УДК 616-092.18(811.111)-054.6(076.5) (075.8) ББК 52.52 (81.2 Англ-923) П20 Рекомендовано Научно-методическим советом университета в качестве практикума 16.09.2015 г., протокол № 1 Авторы: Ф. И. Висмонт, В. А. Касап, С. А. Жадан, А. А. Кривчик, Е. В. Леонова, Т. В. Короткевич, Л. С. Лемешонок, А. В. Чантурия, Т. А. Афанасьева, В. Ю. Перетятько, О. Г. Шуст, Н. А. Степанова, К. Н. Грищенко, Э. Н. Кучук, Д. М. Попутников, Е. В. Меленчук Рецензенты: член-корр. НАН Беларуси, д-р мед. наук, проф. каф. нормальной физиологии Л. М. Лобанок; д-р мед. наук, проф. каф. патологической анатомии М. К. Недзьведзь Патологическая физиология = Pathological physiology : практикум / Ф. И. Вис- П20 монт [и др.]. – 4-е изд. – Минск : БГМУ, 2015. – 191 с. ISBN 978-985-567-304-1. Содержит описания и протоколы оформления лабораторных работ по основным разделам кур- са патофизиологии. Представлена информация по следующим разделам: патофизиология системы крови, нарушение сердечного ритма, кислотно-основного состояния, наследственности и изменчи- вости организма. Первое издание вышло в 2010 году. Предназначен для студентов 2–3-го курсов медицинского факультета иностранных учащихся для самостоятельной подготовки к занятиям, выполнения и оформления лабораторных работ по предмету. УДК 616-092.18(811.111)-054.6(076.5) (075.8) ББК 52.52 (81.2 Англ-923) ISBN 978-985-567-304-1 ã УО «Белорусский государственный медицинский университет», 2015 3 SECTION I GENERAL NOSOLOGY LESSON 1. -
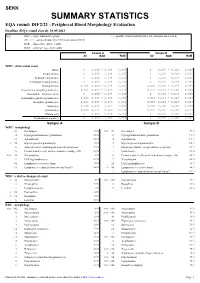
SUMMARY STATISTICS EQA Round: DIF2/21 - Peripheral Blood Morphology Evaluation Deadline (EQA Round Closed): 28.05.2021 Key: ELG
SEKK SUMMARY STATISTICS EQA round: DIF2/21 - Peripheral Blood Morphology Evaluation Deadline (EQA round closed): 28.05.2021 Key: ELG ... expert laboratories group > ... possible result (found by ELG, but consensus not reached) AV, >>> ... assigned value type CVE (consensus of ELG) RAR ... range of acceptable results RoM ... robust average of all results Sample A Sample B AV RAR RoM AV RAR RoM WBC - differential count Blasts 0 0,000 - 0,018 0,000 0 0,000 - 0,018 0,000 Promyelocytes 0 0,000 - 0,018 0,000 0 0,000 - 0,018 0,000 Neutrophil myelocytes 0 0,000 - 0,018 0,000 0 0,000 - 0,018 0,000 Neutrophil metamyelocytes 0 0,000 - 0,018 0,000 0 0,000 - 0,018 0,000 Neutrophil bars 0,0010,000 - 0,018 0,003 0,0020,000 - 0,018 0,004 Segmented neutrophil granulocytes 0,5550,483 - 0,625 0,579 0,5750,503 - 0,644 0,584 Eosinophils - immature forms 0 0,000 - 0,018 0,000 0 0,000 - 0,018 0,000 Eosinophil segmented granulocytes 0,0690,039 - 0,115 0,060 0,0160,003 - 0,043 0,018 Basophilic granulocytes 0,0110,001 - 0,036 0,011 0,0070,000 - 0,028 0,007 Monocytes 0,0660,035 - 0,109 0,067 0,0640,035 - 0,109 0,059 Lymphocytes 0,2980,237 - 0,369 0,278 0,3310,265 - 0,400 0,326 Plasma cells 0 0,000 - 0,018 0,000 0 0,000 - 0,018 0,000 Erythroblasts (number) 0 0,000 - 2,000 0,000 0 0,000 - 3,000 0,002 Sample A Sample B WBC - morphology 23 No changes 15 % >>> 89 No changes 59 % 4 Hypergranulation/toxic granulation 2,6 % 2 Hypergranulation/toxic granulation 1,3 % 10 Agranulation 6,6 % 2 Agranulation 1,3 % > 78 Hypersegmented granulocytes 52 % 3 Hypersegmented granulocytes 2,0 % 6 Atypical/reactive/pathological monocytes/promono. -

Erythronoclastic) Anaemias of Later Infancy and Childhood: with Special Reference to the Acute Heemolytic Anemia of Lederer and the Anvemia of Von Jaksch
Arch Dis Child: first published as 10.1136/adc.8.45.184 on 1 June 1933. Downloaded from Part V.-The hamolytic (erythronoclastic) anaemias of later infancy and childhood: with special reference to the acute heemolytic anemia of Lederer and the anvemia of von Jaksch BY LEONARD G. PARSONS, M.D., F.R.C.P., AND J. C. HAWKSLEY, M.D., M.R.C.P. The destructive diseases of the erythron which fall for consideration under this heading show many points of general similarity although individual variations are frequent. In Part IV emphasis has been laid upon the fact that the erythron may be affected adversely either in the bone marrow or in the circulation; further, that for this reason the term haemolytic anaemia was probably incorrect because it stressed the destruction of red blood cells, but that the title of erythronoclastic ansemia was more correct http://adc.bmj.com/ because it indicated that the erythron may be affected in the circulation or in the marrow or in both situations. Moreover, this is not the whole story, because not only may the erythron itself be affected in either the circulation, the marrow or both, but also the other elements of the blood, the thrombocyte system and the myeloid leucocyte system, may suffer damage. in child's of iron Again, the latter months of the lactation period the supply on September 27, 2021 by guest. Protected copyright. for haemoglobin-building is somewhat precarious; a disturbance of the erythron is therefore likely to render manifest any iron deficiency and thus a deficiency anaemia may develop in the course of a haemolytic ansemia. -

RBC Morphology Normal RBC Morphology
Dr.Iman Daham , BSci., MSc., PhD. Assist. Prof., Department of Internal and Preventive Medicine College of Veterinary Medicine, University of Mosul, Mosul, Iraq https://orcid.org/0000-0002-0947-7169 https://www.researchgate.net/profile/Iman Daham Clinical Pathology | Part I | 4th year 2019 RBC Morphology Normal RBC Morphology: Clinical Pathology | RBC Morphology | Dr. Iman Daham Page | 1 RED BLOOD CELL MORPHOLOGY Abnormal erythrocyte morphology is found in pathological states that may be : - abnormalities in size (anisocytosis). - In shape (poikilocytosis). -In hemoglobin content or the presence of inclusion bodies in erythrocyte. Hypochromic: A descriptive term applied to a red blood cell with a decreased concentration of hemoglobin. Normochromic: A descriptive term applied to a red blood cell with a normal concentration of hemoglobin. Normocytic: A descriptive term applied to normal size of RBC Macrocytic: A descriptive term applied to a larger than normal red blood cell. I-Variation in erythrocyte size (anisocytosis) 1-Microcytosis: Morphology: - Decrease in the red cell size. Red cells are smaller than ± 7µm in diameter. The nucleus of a small lymphocyte (± 8,µm) is a useful guide to the size of a red blood cell. Found in: - Iron deficiency anemia. - Thalassaemia. - Sideroblastic anemia. - Lead poisoning. - Anemia of chronic disease. Comment: Most erythrocytes presented in the picture are microcytes (compare with the small lymphocyte). The degree of hemoglobinization is sufficient. Normal platelets and single ovalocytes are present. Staining: MGG Magnification: x 1000 1. microcyte 2. normocyte 2-Macrocytosis: Morphology: Increase in the size of a red cell. Red cells are larger than 9µm in diameter. May be round or oval in shape, the diagnostic significance being different. -

Red Blood Disorders Anemia
Lviv National Medical University Department of pathological physiology PATHOLOGY OF RED BLOOD PhD. Sementsiv N.G. In norm The number of erythrocytes: in female - 3,9-4,7·1012/l in male - 4,5-5,0·1012/l Hemoglobin in female - 120-140g/l in male - 140-160g/l Color index(CI) - 0,85-1,15 Globular value = 3 x Hb / the first 3 figures of erythrocytes. Reticulocytes - 0.5-2%, 0,5-2%0 changes in total blood volume normovolemia hypovolemia hypervolemia simple (Ht - norm), polycitemia (Ht > 0,48), olygocytemia (Ht < 0,36). A) Norm B) acut anaemia б) acute hemorrhage г) hydremia Pathological forms of erythrocytes regenerative degenerative cell pathologic regeneration Regenerative forms reticulocytes залежно від зрілості розрізняють: (Зернисті) Stippling (Сітчасті) Mesh Norm in a blood reticulocytes - 0,2–2,0%. Regenerative forms Basophiles substantial erythrocytes - cytoplasm remains basophilic normo blast. Polychromatophil erythrocytes (polychromasia, polychromatophilia ) – erythrocytes with basophiles substantial ( blue cells) indicates increased RBC production by the marrow Qualitative (degenerative) changes of red blood cell - poikilocytosis - different shape of erythrocytes; - anisocytosis - different size of erythrocytes; - anisochromia - different saturation of red blood cells by hemoglobin Degenerative forms Anisocytosis present in a blood different forms erythrocytes » normocyte (7,01–8,0 мкм) » microcyte(6,9–5,7 мкм) » macrocyte(8,1–9,35 мкм) » megalocyte (10–15 мкм) Degenerative forms Poikilocytosis present in a blood pictures erythrocytes different forms : elongate form , oval, ellipsoid and os. ОVAlOCYTE( ELLIPTOCYTE) – 5% all blood. Pathological cells regenerate Megaloblast mehaloblastyc cell type hematopoiesis Megaloblast oval cells in the diameter of 1,5- 2,0 times larger than normal erythrocytes is the final stage mehaloblastyc hematopoiesis. -
Clinical Experience I, Ii, Iii, Iv C
CLINICAL EXPERIENCE I, II, III, IV Revised 5/5/04 C - L -CR COURSE NUMBER: MLT 270 0 - 36 - 12 MLT 251, MLT 252 0 - 15 - 5 PREREQUISITE(S): Successful completion of earlier program requirements CO-REQUISITE(S): None COURSE This course provides an integrated, clinically-based rotation DESCRIPTIONS which correlates cognitive and technical skills in selected areas of the clinical laboratory. TEXTBOOK(S): Ciulla, Anna P. and Georganne K. Buescher. Q & A Review Medical Technology/Clinical Laboratory Science. 3rd ed. New Jersey: Prentice Hall, 2002. Polansky, Valerie D. Quick Review Cards for Laboratory Science Examinations. Philadelphia: F. A. Davis Company, 2000. Leach, Donna L and G. Ryman Denny (eds). Outline Review Medical Technology/Clinical Laboratory Science. New Jersey: Prentice Hall, 2004. REFERENCE(S): None OTHER REQUIRED Students will use the Computer Lab to perform computer MATERIALS, TOOLS, reviews, tutorials, and practice tests. AND EQUIPMENT: METHOD OF The student will use the self-paced texts to answer study INSTRUCTION: questions and take tests. Computer reviews will also be used in the course. Students will write journal article summaries to identify recent technological changes and emerging technologies. GRADING SYSTEM: 94 - 100= A 86 - 93= B 80 - 85= C 75 - 79= D Below - 74 = F 2 GRADING SYSTEM An overall 80% or better average is required to pass each of CONTINUED: the clinical rotations (courses). Final grade averages will not be rounded. GRADE There are three clinical courses: MLT 270, MLT 251, and CALCULATION MLT 252. Each course contains departmental rotations in METHOD: Chemistry, Blood Bank, Microbiology (to include Serology and Parasitology), Hematology (to include Coagulation), Urinalysis, Phlebotomy Services, Physician's Office/Other, and 3-11 rotation. -
A Laboratory Guide to Clinical Hematology
A Laboratory Guide to Clinical Hematology A Laboratory Guide to Clinical Hematology A Laboratory Guide to Clinical Hematology VALENTIN VILLATORO AND MICHELLE TO EDMONTON A Laboratory Guide to Clinical Hematology by Michelle To is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, except where otherwise noted. Please be aware that the content for the entirety of this eBook is subject to a creative common license: Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) You are free to: Share — copy and redistribute the material in any medium or format Adapt — remix, transform, and build upon the material The licensor cannot revoke these freedoms as long as you follow the license terms. Under the following terms: Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. NonCommercial — You may not use the material for commercial purposes. No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits. Contents Authors & Editors ................................................................................................................................... xii Creative Commons License and Citation ............................................................................................... xiii Contact Information and Feedback ........................................................................................................