Residual Normal B-Cell Proiles in Monoclonal B-Cell Lymphocytosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gastronomy of Salamanca
gastronomy of Salamanca 1 Salamanca is a province to enjoy with all your sen- ses, including your taste buds. The province has an excellent and varied range of raw produce, with some well-known, quality products among them. Iberian ham and cold cuts, beef, pulses, hornazo, cheese and wines are some of the most traditional ones, which in the hands of master chefs, become succulent dishes. Come, look, try and taste the varied, delicious menu that Salamanca has prepared for you. 2 His Majesty the Iberian Pig The Iberian pig is a unique, fascinating breed of un- questionable quality, which puts the Salamanca pro- vince as an international benchmark. Its pedigree, along with the long walks in search for food, are responsible for the trademark infiltrated fat, which results in its flavour, its aroma and juici- ness, as well as its health benefits, since this fat is high in oleic acids. This pig lives free in the Salamanca dehesa, an id- yllic environment where, between October and February, it enjoys the best, final fattening seasons when it is fed only with acorns. 3 Guijuelo and the neighbouring counties Talking about Iberian produce is talking about Guijuelo, a unique place with a climate and conditions that are ideal for curing hams of all sorts as well as cold cuts. The long, cold, dry winters, along with its moody winds and humid conditions, mean that less salt is required for the curing process. This enables their flavours, aromas and uniqueness to be expressed in a much more natural way. And although Guijuelo is the heart of the pork product manufacturing in Salamanca, the actual production area includes other municipalities, some as well known as La Alberca, Béjar, Candelario or San Martín del Castañar. -

165 Lunes 27
N.º 165 • Lunes 27 de Agosto de 2018 Pág. 1 IV. Administración Local OTROS ENTES LOCALES REGTSA Organismo Autónomo Recaudación y Gestión Tributaria Salamanca ANUNCIO DE COBRANZA Se pone en conocimiento de los contribuyentes y demás interesados de la provincia, que desde el Día 1 de septiembre de 2.018 hasta el día 31 de Octubre de 2.018, ambos inclusive, tendrá lugar la cobranza en periodo voluntario de los siguientes tributos: • Impuesto sobre Bienes Inmuebles de naturaleza Urbana.(Segundo Plazo en los si- guientes ayuntamientos: La Alberca, Aldealengua, Aldeatejada, Buenavista, Candelario, Car- bajosa de la Sagrada, Carrascal de Barregas, Ciudad Rodrigo, Doñinos de Salamanca, Fuen- teguinaldo, Fuentes de Oñoro, Lagunilla, Macotera, Miranda de Azan, Mogarraz, Moriscos, Mo- zarbez, Peñaranda de Bracamonte, San Cristóbal de la Cuesta, San Pedro de Rozados, Santa Marta de Tormes, Tamames, Terradillos, Torresmenudas, Valdelosa, Villares de la Reina , Vi- lloria y Villoruela). • Impuesto sobre Actividades Económicas • Tasas y precios públicos en los Municipios que se reflejan en el Anexo I. Las listas cobratorias de las Tasas y Precios Públicos cuya cobranza se efectúa en el pre- sente período voluntario de Recaudación, correspondientes a los Ayuntamientos que tienen en- comendada la Gestión Tributaria a este Organismo, estarán expuestas al público en la Secre- taría de los mismos y en las cabeceras de las Zonas correspondientes, durante el plazo de TREINTA DÍAS contados desde el siguiente al de la publicación de este anuncio en el Boletín Oficial de la Provincia (Art. 9-2 de la Ordenanza General de gestión y recaudación de REGTSA, B.O.P. -

Demarcaciones Asistenciales De Enfermería Área De Salamanca
ANEXO I I - DEMARCACIONES ASISTENCIALES DE ENFERMERÍA ÁREA DE SALAMANCA C.S - Consultorio Núcleo de población Nº 1 Salamanca Nº 2 Salamanca Nº 3 Salamanca Nº 4 Salamanca Nº 5 Salamanca Nº 6 Salamanca Nº 7 Salamanca ZBS ALAMEDILLA Nº 8 Salamanca Nº 9 Salamanca Nº 10 Salamanca Nº 11 Salamanca Nº 12 Salamanca Nº 13 Salamanca Nº 14 Salamanca Nº 1 Salamanca Nº 6 Salamanca Nº 13 Salamanca ZBS CAPUCHINOS Nº 14 Salamanca Nº 16 Salamanca Nº 17 Salamanca Nº 1 Salamanca Nº 2 Salamanca Nº 3 Salamanca Nº 4 Salamanca Nº 5 Salamanca Nº 6 Salamanca ZBS GARRIDO NORTE Nº 7 Salamanca Nº 8 Salamanca Nº 9 Salamanca Nº 10 Salamanca Nº 11 Salamanca Nº 12 Salamanca Nº 13 Salamanca Nº 1 Salamanca Nº 2 Salamanca Nº 3 Salamanca Nº 4 Salamanca Nº 5 Salamanca Nº 6 Salamanca Nº 7 Salamanca Nº 8 Salamanca Nº 9 Salamanca ZBS GARRIDO SUR Nº 10 Salamanca Nº 11 Salamanca Nº 12 Salamanca Nº 13 Salamanca Nº 14 Salamanca Nº 15 Salamanca Nº 16 Salamanca Nº 17 Salamanca Nº 18 Salamanca Nº 2 Salamanca Nº 3 Salamanca Nº 4 Salamanca Nº 5 Salamanca Nº 7 Salamanca ZBS PIZARRALES-VIDAL Nº 8 Salamanca Nº 9 Salamanca Nº 10 Salamanca Nº 11 Salamanca Nº 12 Salamanca Nº 15 Salamanca Nº 1 Salamanca Nº 2 Salamanca Nº 3 Salamanca Nº 4 Salamanca Nº 5 Salamanca Nº 6 Salamanca ZBS SAN BERNARDO OESTE Nº 7 Salamanca Nº 8 Salamanca Nº 9 Salamanca Nº 10 Salamanca Nº 11 Salamanca Nº 12 Salamanca Nº 13 Salamanca Nº 1 Salamanca Nº 2 Salamanca Nº 3 Salamanca Nº 4 Salamanca ZBS SAN JOSÉ Nº 5 Salamanca Nº 6 Salamanca Nº 7 Salamanca Nº 8 Salamanca Nº 9 Salamanca Nº 1 Salamanca Nº 2 Salamanca Nº 3 Salamanca -
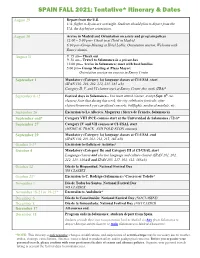
Tentative* Itinerary & Dates
SPAIN FALL 2021: Tentative* Itinerary & Dates August 29 Depart from the U.S. U.S. flights to Spain are overnight. Students should plan to depart from the U.S. the day before orientation. August 30 Arrive in Madrid and Orientation on safety and program polices 12:00 – 5:00 pm-- Check in at Hotel in Madrid 6:00 pm--Group Meeting at Hotel Lobby. Orientation session, Welcome with Emory alumni. August 31 9: 15 am-- Check out 9: 30 am-- Travel to Salamanca in a private bus 12:00 pm-- Arrive in Salamanca; meet with host families 5:00 pm-- Group Meeting at Plaza Mayor: Orientation session on courses in Emory Center September 1 Mandatory (Category Ia) language classes at CI-USAL start (SinPAN Emory 102, Center.201, 202, Orientation 212, 215, 3 Session85 a/b) on courses. Category II, V, and VI classes start at Emory Center this week (TBA)* September 6-12 Festival days in Salamanca - You must attend classes, except Sept. 8th (no classes) Note that during this week, the city celebrates festivals; after classes/homework you can attend concerts, bullfights, medieval markets, etc. September 26 Excursion to La Alberca, Mogarraz ( Sierra de Francia, Salamanca) September end* Category VIII (PCI) courses start at the Universidad de Salamanca (TBA)* September 27 Category IV and VII courses at CI-USAL start (MEDICAL TRACK AND POLS/ ECON courses) September 29 Mandatory (Category Ia) language classes at CI-USAL end (SPAN 102, 201, 202, 212, 215, 385 a/b) October 1-3* Excursion to Galicia or Asturias* October 4 Mandatory (Category Ib) and Category III at CI-USAL start Language classes and elective language and culture classes (SPAN 201, 202, 212, 215, 310A/B and SPAN 205, 217, 302, 312, 385a/b) October 12 Día de la Hispanidad, National Festival Day NO(SPAN CLAS 202,SES 212, 310,etc) October 23* Excursion to C. -

Senderismo En Salamanca
senderismo en Salamanca 1 2 ¡en marcha! Existen muchas formas de conocer Salamanca y una de las más atractivas, sin duda, es ca- minando. Deporte, cultura y medio ambiente se combinan en la práctica del senderismo, posible gracias a las rutas señalizadas que discurren por distintos puntos de la provincia. De largo o pequeño recorrido, estos caminos ofrecen escenarios únicos para descubrir el territorio, el patrimonio histórico y cultural, el entorno natural y la forma de vida de los que habitaron y habitan estas tierras. Salamanca ofrece rutas que permiten disfrutar de espacios naturales con inmenso valor ecológico como las Sierras de Francia y de Béjar y el Parque Natural Arribes del Duero, declarados por la UNESCO como Reservas de la Biosfera. Las sierras de Béjar y de Francia ocupan casi 200.000 hectáreas, lo que las convierte en la mayor Reserva de la región y la tercera de toda España. En ellas se encuentra el parque natural de las Batuecas-Sierra de Francia y las Quilamas, espacios naturales que se pue- den recorrer a través de los itinerarios propuestos. Caminos mágicos inmersos también en el parque natural de Arribes del Duero, que forma parte de la Reserva “Meseta Ibérica”, el mayor con carácter transfronterizo de Europa. Y en torno a estos espacios verdes, poblaciones con encanto. Lugares donde pasear sin prisas y hacer una pausa para contemplar las vistas, descansar y degustar cualquiera de los productos y platos típicos que solo ofrecen estas tierras. ¡En marcha! 3 Caminos de Arte en la Naturaleza Cuatro sugestivos senderos ubicados en la atractiva comarca de la Sierra de Francia integran los llamados Caminos de Arte en la Naturaleza. -

Ministerio De Economía Yhacienda Ministerio Del
Boe núm. 285 Viernes 28 noviembre 1997 21367 MINISTERIO MINISTERIO DEL INTERIOR MINISTERIO DE FOMEN¡O DE ECONOMÍA YHACIENDA Resolución de la Dirección General de T,.qj"ICo Resolación de la Secretaria de Estado de por la que se COnJ1f1Ca concurso abierto para Infraestructuras y Transportes por la que Resolución de la Delegación Provincial de suministra para la ampliación de' los sis se anuncia la licitación de contrlltos de Salamanca. Gerencia Territorial de Catas temas audiotexto en los Centras de Gestión obras, por el procedimiento abierto yforma Ú'O, por'la que se hace pública la adjudi de TráfICO de Sevilla y Málaga. de adjudicación de subasta. cación de los trabajos catastrales que se citan, incluidos en los expédientes l. Entidad adjudicadora: DISPOSICIONES COMUNES 0597RU372, 0697RU372, 0797RU372 y 1. Entidad adjudicadora: 0897RU372. a) Organismo: Dirección General de Tráfico. b) Dependericia'que tramita el expediente: Ser- a) Organismo: Secretaria de Estado de infraes 1. Entidad adjudicadora: vicio de Inversiones. 1 .' - tructurasy Transportes. Dirección General de Carre teras. a) Organismo: Delegación Provincial Eco e) Número de expediente: 8-80-60083-2. de b) Dependencia que tramíta el expediente: nomía y Hacienda de Salamanca (expedientes Secretaria General. 0597RU372y 0697RU372) y Consejo Territorial 2. Objeto del contrato: de la Propiedad Inmobiliaria de Salamanca (expe a) Descripción del objeto: Suministro para la 2. Tramitación, procedimiento y forma de adju dientes 0797RU372 y 0897RU372). ampliación de los sistemas audiotexto'en los Centros dicación: b) Dependencia que tramita los expedientes: de Gestión de Tráfico de Sevilla y Málaga a) Tramitación: Ordinaria. Gerencia Territorial del Catastro de Salamanca b) Número de unidades a entregar: Dos. -

Organización Para La Atención Desde Los Servicios Sociales Ante La Situación De Emergencia Covid 19
ORGANIZACIÓN PARA LA ATENCIÓN DESDE LOS SERVICIOS SOCIALES ANTE LA SITUACIÓN DE EMERGENCIA COVID 19 ÁREA SEGUNDA SEDE de UBICACIÓN: DEPENDENCIAS CENTRALES DEL ÁREA TFNO.: 923/217410 correo electrónico: DE BIENESTAR SOCIAL [email protected] CEAS ALBA DE TORMES CEAS PEÑARANDA CEAS VILLAS CEAS RURAL NORTE CEAS RURAL SUR Aldeaseca de Alba Rágama Negrilla de Palencia Santiz Monterrubio de la Sierra Fresno Alhándiga Malpartida Orbada (La) Palacios del Arzobispo Canillas de Abajo Galinduste Macotera Cantalpino Almenara de Tormes Miranda de Azán Sieteiglesias de Tormes Alaraz Espino de la Orbada Añover de Tormes Aldehuela de la Bóveda Valdecarros Tordillos Aldeanueva de Figueroa San Pelayo de Guareña Aldeatejada Anaya de Alba Bóveda del Río Almar Arabayona de Mógica Villares de la Reina Morille Gajates Mancera de Abajo Villoria Arco (El) Robliza de Cojos Buenavista Ventosa del Río Almar Pajares de la Laguna Zamayón Galindo y Perahuy Encinas de Arriba Salmoral Villaverde de Guareña Forfoleda San Pedro de Rozados Pedrosillo de los Aires Nava de Sotrobal Vellés (La) Valdelosa Mozárbez Pedraza de Alba Santiago de la Puebla Parada de Rubiales Moriscos Barbadillo Alba de Tormes Alconada Pedroso de la Armuña (El) Torresmenudas Arapiles Peñarandilla Paradinas de San Juan Tardáguila Calzada de Valdunciel Matilla de los Caños del Río Pedrosillo de Alba Poveda de las Cintas Pedrosillo el Ralo Monterrubio de Calzada de Don Diego Armuña Éjeme Aldeaseca de la Frontera Arcediano Valdunciel Vecinos Pelayos Cantaracillo Pitiegua Castellanos -

DATOS ACERCA DE LA FLORA SALMANTINA Por FRANCISCO AMICH GARCÍA*
DATOS ACERCA DE LA FLORA SALMANTINA por FRANCISCO AMICH GARCÍA* Resumen Amich García, F. (1980). Datos acerca de la Flora Salmantina. Anales Jará. Bot. Madrid 36: 291-300. Se dan a conocer algunas novedades corológicas de interés varío, relaciona das, todas ellas, con la comarca de Vitigudino, objeto de nuestra Memoria Doc toral. Destacaremos como más importante la primera cita en España de: Trigo nella polyceratia L. var. longipes Sampaio y Lamium moluccellifolium Fries. Abstract Amich García, F. (1980). Some data on the Flora of Salamanca. Anales Jard. Bot Madrid 36: 291-300 (In Spanish). In the present work we describe some new chorologic findings for the Iberian Flora and specially related to the Vitigudino área; this work was the object of our Doctoral dissertation and represents a contribution towards a better kno- wledge of their distribution. We also indícate two new records for the Spanish Flora: Trigonella polyceratia L. var. longipes Sampaio and Lamium moluccellifolium Fries. Con motivo del estudio de la flora y vegetación de la comarca de Vitigudino (Salamanca), objeto de nuestra Memoria Doctoral, hemos lo-, calizado algunas plantas cuyo areal, en la península, no era bien cono cido. Los testimonios de las citaciones se encuentran depositados en el Herbario del Departamento de Botánica de la Facultad de Biología, al que nos referiremos con las siglas SA, todavía no incluidas en el Index Herbariorum. * Departamento de Botánica. Facultad de Biología. Salamanca. 292 ANALES DEL JARDÍN BOTÁNICO DE MADRID Arabis lusitanica Boiss., Diagn. Pl. Or. Nov. 3(1):20(1853). La hemos recolectado en enclaves arenosos muy ruderalizados a ori llas del Duero, como: Corporario, 16-IV-1978, F. -

Dirección Provincial De Salamanca
ORDEN EDU/204/2017, DE 17 DE MARZO, DE SELECCIÓN Y NOMBRAMIENTO DE DIRECTORES ANEXO I LISTADO DEFINTIVO DE ADMITIDOS DIRECCIÓN PROVINCIAL DE SALAMANCA CENTRO Nº APELLIDOS NOMBRE LOCALIDAD Nº TIPO DENOMINACIÓN ESPECÍFICA 1 BARRANQUERO MONTES M BELEN 1 CEIP MIGUEL DE CERVANTES GUIJUELO 2 BARRIOS PEREZ JULIAN 1 IES RAMON OLLEROS GREGORIO BEJAR 3 BLANCO BALLESTEROS JESUS FRANCISCO 1 CEPA MATEO HERNANDEZ BEJAR 4 BENITO DE DIOS JUAN ANTONIO 1 CEIP SAN MATEO SALAMANCA 5 CALVO RODRIGUEZ MARCIAL 1 CEIP FELIX RODRIGUEZ DE LA FUENTE SALAMANCA 6 COLORADO MUÑOZ M DE LAS MERCEDES 1 CEIP LA ANTIGUA BEJAR 7 CRUZ HORTAL JOSE ANDRES 1 IES TIERRA DE CIUDAD RODRIGO CIUDAD RODRIGO 8 GARCIA BELLIDO M DEL CARMEN 1 CEIP VILLA DE FELIPE II VILLORUELA 9 GARCIA MARTIN ALEJANDRO 1 EA ESCUELA DE ARTE SALAMANCA 10 GONZALEZ CASTRILLO MIGUEL ANGEL 1 CEIP PIEDRA DE ARTE VILLAMAYOR 11 GONZALEZ LOPEZ AGUSTIN 1 CRA ABADENGO HINOJOSA DE DUERO 12 HERNANDEZ BEJARANO MAGIN 1 CRA ARMUÑA PEDROSILLO EL RALO 13 HERNANDEZ PEREZ ROSA MARIA 1 CRA LOS CARRASCALES MATILLA DE LOS CAÑOS DEL RIO 14 HOLGADO CABALLERO M TERESA 1 CEIP RUFINO BLANCO SALAMANCA 15 IGLESIAS DOMINGUEZ JOSEFA M 1 CRA LOPE DE VEGA GARCIHERNANDEZ 16 MARTIN BARRADO SIMON PASCUAL 1 IES VAGUADA DE LA PALMA SALAMANCA 17 MARTIN HIDALGO MARIA 1 CEIP FILIBERTO VILLALOBOS BEJAR 18 MARTIN YENES ASUNCION 1 CEIP SEVERIANO MONTERO SANCHEZ PEÑARANDA DE BRACAMONTE 19 MORENO HERNANDEZ MANUEL 1 CEPA JOSE TOMAS DE MAZARRASA CIUDAD RODRIGO 20 PEREZ SANCHEZ M JOSE 1 CRA LOS JARALES SAN MIGUEL DE VALERO 21 RAMOS ARRANZ M DEL CARMEN -

N.º 134 • Miércoles 14 De Julio De 2021
N.º 134 • Miércoles 14 de Julio de 2021 SE PUBLICA TODOS LOS DÍAS EXCEPTO SÁBADOS, DOMINGOS Y FESTIVOS Administración: Excma. Diputación Provincial de Salamanca. Domicilio: Felipe Espino, 1. Teléf.-Fax 923 29 31 35. [email protected] D.L.: S. 1-1958. ADVERTENCIA EDITORIAL.- Todas las inserciones en el Boletín Oficial de la provincia de Salamanca se regirán por lo establecido en el Reglamento de Gestión del Boletín Ofi- cial de la provincia de Salamanca (B.O.P. nº 89 de 13 de mayo de 2010) y por la Orde- nanza Fiscal Reguladora de la Tasa por la prestación de los servicios del Boletín Oficial de la provincia de Salamanca (B.O.P. nº 20, de 11 de febrero de 2010). PRESENTACIÓN DE ORIGINALES.- Los usuarios del Boletín Oficial de la provincia están obligados a presentar los originales tanto en copia impresa como en formato digital (preferiblemente realizados en cualquier programa de tratamiento de texto o en formato PDF abierto). Ambos originales deben ser copia exacta en los contenidos. https://sede.diputaciondesalamanca.gob.es/BOP/ N.º 134 • Miércoles 14 de Julio de 2021 Administración Autonómica. –JUNTA DE CASTILLA Y LEÓN. Delegación Territorial de Salamanca. Servicio Territorial de Medio Ambiente. ANUNCIO DE INFORMACIÓN PÚBLICA DE EXPEDIENTE DEL PROCEDIMIENTO DE PRÓRROGA DEL COTO DE CAZA SA-11533. CVE: BOP-SA-20210714-001 ANUNCIO DE INFORMACIÓN PÚBLICA DE EXPEDIENTE DEL PROCEDIMIENTO DE CAMBIO DE TI- TULARIDAD DEL COTO DE CAZA SA-11453. CVE: BOP-SA-20210714-002 Administración Local. –AYUNTAMIENTOS. Cabrerizos. Edicto de notificación colectiva de liquidaciones y anuncio de cobranza. -

Maquetación 1
await you. await and its emotions emotions its and Salamanca experiences for sharing, sharing, for experiences Proposals for feeling, feeling, for Proposals (MTB) routes and centres, and skiing at La Cova5lla. La at skiing and centres, and routes (MTB) Dehesa, discovering landscapes thanks to the mountain bike mountain the to thanks landscapes discovering Dehesa, Art in Nature, exploring the Route of the Figh5ng Bull and the and Bull Figh5ng the of Route the exploring Nature, in Art Other recommenda5ons will have you walking the Routes of Routes the walking you have will recommenda5ons Other the len5ls of La Armuña. Armuña. La of len5ls the of Salamanca, the cheeses of Las Arribes, the olive oil, and oil, olive the Arribes, Las of cheeses the Salamanca, of the wines of the Sierra de Francia and Las Arribes, the meats the Arribes, Las and Francia de Sierra the of wines the cap5vated by tasty tempta5ons such as the Ham of Guijuelo, of Ham the as such tempta5ons tasty by cap5vated or you can be a real pilgrim on the Via de la Plata. You will be will You Plata. la de Via the on pilgrim real a be can you or Passions, the Corpus Chris5, the pilgrimages and offertories,... and pilgrimages the Chris5, Corpus the Passions, Carnival of the Bull and its running of the bulls, the Living the bulls, the of running its and Bull the of Carnival You will also feel the vibra5ons of the folk tradi5ons: the tradi5ons: folk the of vibra5ons the feel also will You Historical Ensembles, and the Fron5er For5fica5ons. -

La Vida Consagrada En Salamanca En La Época De Barbado Viejo (1943-1964)
Salmanticensis 59 (2012) 243-289 La vida consagrada en Salamanca en la época de Barbado Viejo (1943-1964) Eduardo Javier Alonso Romo Universidad de Salamanca; Facultad de Filología (Portugués) Resumen: En el presente tra- Summary: In the present work bajo nos acercamos a la vida consa- we approach the consecrated life grada en la diócesis de Salamanca in the diocese of Salamanca along a lo largo de los veintiún años en twenty-one years in which this one que ésta es regida por el obispo is governed by the bishop Domi- dominico Fr. Francisco Barbado nican Fr. Francisco Barbado Viejo Viejo (1943-1964). Se trata de car- (1943-1964). One treats of chart the tografíar las presencias institucio- institutional presences –specially nales –especialmente las nuevas the new foundations– and of some fundaciones– y de señalar algunos significant prominent figures indi- personajes significativos a lo largo cate along this period inside reli- de este periodo dentro de los reli- gious and consecrated, with special giosos y consagrados, con especial attention to that they were uni- atención a los que fueron profeso- versity teachers, given the narrow res universitarios, dada la estrecha entail of this prelate with the Uni- vinculación de este prelado con la versity Pontificia. Universidad Pontificia. Palabras clave: Francisco Bar- Keywords: Francisco Barbado bado Viejo, institutos religiosos, Viejo, religious institutes, Pontifical Universidad Pontificia de Sala- University of Salamanca, vocations, manca, vocaciones, cartografía urban cartography, twentieth cen- urbana, siglo XX. tury. Universidad Pontificia de Salamanca 244 EDUARDO Javier ALONSO ROMO 1. INT R ODUCCIÓN Próximo ya a cumplirse el medio siglo desde su fallecimiento, el tiempo y la figura del obispo Fray Francisco Barbado Viejo nos pueden parecer bastante lejanos1, como anteriores que son a la profunda renovación propiciada por el Concilio Vaticano II y a las crisis de todo tipo que vinieron inmediatamente después y que afec- taron de modo particular a la vida consagrada.