A Study on Groundwater Quality in the Pondicherry Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Villupuram - District Agricultural Plan
Villupuram - District Agricultural Plan Project team Preface Foreword Executive Summary Chapter I Chapter II Chapter III Chapter IV Chapter V Chapter VI Meeting Proceedings Photos NATIONAL AGRICULTURE DEVELOPMENT PROJECT – DISTRICT AGRICULTURE PLAN PROJECT TEAM Overall Coordination : Dr. K. Palanisami, Director, CARDS and Nodal Officer (NADP) Dr. R. Venkatram, Professor and Principal Coordinator (NADP) District Level : Mrs. S. Hemalatha Coordination Assistant Professor Dept. of ARM, TNAU, Coimbatore Dr.G.Rangaraju Professor and Head Oilseed Research Station Tindivanam Dr. C. Velevan Assistant Professor Oilseed Research Station Tindivanam Mr. B. Kadarmaideen Executive Engineer (AED) Villupuram Mr. S. Rajasekaran Deputy Director of Horticulture Villupuram Mr. S. Palanivel Assistant Engineer (AED) Villupuram Mr. S. Arunagiri Assistant Engineer Public Works Department Kallakurichi Mr. T. Subramanian, Agricultural Officer Office of the Joint Director of Agriculture Villupuram Tamil Nadu Agricultural University Prof. C.RAMASAMY COIMBATORE-641 003 Vice-Chancellor TAMIL NADU INDIA. FOREWORD Date ........................... The National Development Council resolved that Agricultural Development strategies must be reoriented to meet the needs of farmers and called upon the Central and State governments to evolve a strategy to rejuvenate agriculture with a commitment to achieve four per cent annual growth in the agricultural sector during the 11th plan. The council also recommended special Additional Central Assistance Scheme named National Agriculture Development Programme (NADP) be launched. To implement this, formulation of District level action plans is the pre-requisite and thus District Agriculture Plan of various districts in Tamil Nadu has been prepared with the financial assistance of Government of India. The task of preparing the District Agriculture Plan has been given to Tamil Nadu Agricultural University by Government of Tamil Nadu. -

Mint Building S.O Chennai TAMIL NADU
pincode officename districtname statename 600001 Flower Bazaar S.O Chennai TAMIL NADU 600001 Chennai G.P.O. Chennai TAMIL NADU 600001 Govt Stanley Hospital S.O Chennai TAMIL NADU 600001 Mannady S.O (Chennai) Chennai TAMIL NADU 600001 Mint Building S.O Chennai TAMIL NADU 600001 Sowcarpet S.O Chennai TAMIL NADU 600002 Anna Road H.O Chennai TAMIL NADU 600002 Chintadripet S.O Chennai TAMIL NADU 600002 Madras Electricity System S.O Chennai TAMIL NADU 600003 Park Town H.O Chennai TAMIL NADU 600003 Edapalayam S.O Chennai TAMIL NADU 600003 Madras Medical College S.O Chennai TAMIL NADU 600003 Ripon Buildings S.O Chennai TAMIL NADU 600004 Mandaveli S.O Chennai TAMIL NADU 600004 Vivekananda College Madras S.O Chennai TAMIL NADU 600004 Mylapore H.O Chennai TAMIL NADU 600005 Tiruvallikkeni S.O Chennai TAMIL NADU 600005 Chepauk S.O Chennai TAMIL NADU 600005 Madras University S.O Chennai TAMIL NADU 600005 Parthasarathy Koil S.O Chennai TAMIL NADU 600006 Greams Road S.O Chennai TAMIL NADU 600006 DPI S.O Chennai TAMIL NADU 600006 Shastri Bhavan S.O Chennai TAMIL NADU 600006 Teynampet West S.O Chennai TAMIL NADU 600007 Vepery S.O Chennai TAMIL NADU 600008 Ethiraj Salai S.O Chennai TAMIL NADU 600008 Egmore S.O Chennai TAMIL NADU 600008 Egmore ND S.O Chennai TAMIL NADU 600009 Fort St George S.O Chennai TAMIL NADU 600010 Kilpauk S.O Chennai TAMIL NADU 600010 Kilpauk Medical College S.O Chennai TAMIL NADU 600011 Perambur S.O Chennai TAMIL NADU 600011 Perambur North S.O Chennai TAMIL NADU 600011 Sembiam S.O Chennai TAMIL NADU 600012 Perambur Barracks S.O Chennai -

3040 Tamil Nadu Public Service Commission Bulletin [August 16, 2016
3040 TAMIL NADU PUBLIC SERVICE COMMISSION BULLETIN [AUGUST 16, 2016 DEPARTMENTAL EXAMINATIONS MAY 2016 DEPARTMENTAL TEST IN THE TAMIL NADU MEDICAL CODE (WITH BOOKS) LIST OF REGISTER NUMBER OF PASSED CANDIDATES - CONTD. CHENNAI - Contd. CHENNAI - Contd. 000959 EZHUMALAI V S/O VELLAIYAN, NO.218, MEL ST 001258 INDUMATHI G. MADRAS MEDICAL COLLEGE CHENNAI KOTTAPUTHUR PO, CHINNASALEM TK VILLUPURAM DT PINCODE:600003 PINCODE:606209 001260 INDUMATHI P B4 SHYAMS ROYAL ENCLAVE 25 SATHYA 000963 FARZANA . Y 228/3, MOSQUE STREET BALUCHETTY NAGAR 2ND STREET MOGAPPAIR ROAD PADI CHATRAM KANCHIPURAM PINCODE:631551 PINCODE:600050 000967 FELCY EMALDA M NO.53, MUTHURAMALINGAM ST, 001266 ISAKKIAMMAL .M ROYAL WOMENS HOSTEL 2, SENTHIL NAGAR, THIRUMULLAIVOYAL, CHENNAI VEERASAMY STREET EGMORE CHENNAI. PINCODE:600062 PINCODE:600008 000969 FRANCIS RAJESH A O/O THE GOVERNMENT ANALYST 001300 JANAGHI.M NURSES QUARTERS GOVT STANLEY FOOD ANALYSIS LAB, KI CAMPUS GUINDY CHENNAI - 32 HOSPITAL CHENNAI PINCODE:600001 PINCODE:600032 001314 JASMINE BEAULA D 2/917, NELLI NAGAR NEAR RS WATET 000977 GANAPATHY V 3/340 CHOKKAMMAN KOIL STREET TANK DHARMAPURI PINCODE:636701 DESUMUGIPET POST, THIRUKKALUKUNDRAM PINCODE:603109 001395 JEEVA B 1/56 VINAYAGAR KOIL STREET PUDUMAVILANGAI TIRUVALLUR PINCODE:631203 001012 GAYATHRI C R 49,SUNDARAM STREET STUARTPET ARAKKONAM PINCODE:631001 001396 JEEVA E 42C, MANDAPAM STREET PILLAIYARPALAYAM KANCHIPURAM PINCODE:631501 001033 GEETHA T S7A,EAST MAIN ROAD, LAKSHMI NAGAR 4THSTAGE NANGANALLUR CHENNAI PINCODE:600061 001400 JEEVANAKUMARI A PLOT 20 MIG2 TAMIL NADU HOUSINGBOARD COLONY TONDIARPET PINCODE:600081 001039 GEETHAMAI T. G. N. OLD14/NEW18 DR,RATHAKRISHNAN NAGAR 1ST ST CHOOLAIMEDU,CHENNAI PIN:600094 001418 JEYAKANNAN M. 6-1-69, KATCHAKARIAMMAN KOVIL T.KALLUPATTI PERAIYUR TK, MADURAI DT 001056 GIRIJA P. -
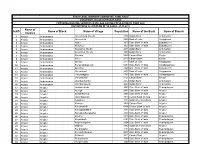
S.NO Name of District Name of Block Name of Village Population Name
STATE LEVEL BANKERS' COMMITTEE, TAMIL NADU CONVENOR: INDIAN OVERSEAS BANK PROVIDING BANKING SERVICES IN VILLAGE HAVING POPULATION OF OVER 2000 DISTRICTWISE ALLOCATION OF VILLAGES -01.11.2011 Name of S.NO Name of Block Name of Village Population Name of the Bank Name of Branch District 1 Ariyalur Andiamadam Anikudichan (South) 2730 Indian Bank Andimadam 2 Ariyalur Andiamadam Athukurichi 5540 Bank of India Alagapuram 3 Ariyalur Andiamadam Ayyur 3619 State Bank of India Edayakurichi 4 Ariyalur Andiamadam Kodukkur 3023 State Bank of India Edayakurichi 5 Ariyalur Andiamadam Koovathur (North) 2491 Indian Bank Andimadam 6 Ariyalur Andiamadam Koovathur (South) 3909 Indian Bank Andimadam 7 Ariyalur Andiamadam Marudur 5520 Canara Bank Elaiyur 8 Ariyalur Andiamadam Melur 2318 Canara Bank Elaiyur 9 Ariyalur Andiamadam Olaiyur 2717 Bank of India Alagapuram 10 Ariyalur Andiamadam Periakrishnapuram 5053 State Bank of India Varadarajanpet 11 Ariyalur Andiamadam Silumbur 2660 State Bank of India Edayakurichi 12 Ariyalur Andiamadam Siluvaicheri 2277 Bank of India Alagapuram 13 Ariyalur Andiamadam Thirukalappur 4785 State Bank of India Varadarajanpet 14 Ariyalur Andiamadam Variyankaval 4125 Canara Bank Elaiyur 15 Ariyalur Andiamadam Vilandai (North) 2012 Indian Bank Andimadam 16 Ariyalur Andiamadam Vilandai (South) 9663 Indian Bank Andimadam 17 Ariyalur Ariyalur Andipattakadu 3083 State Bank of India Reddipalayam 18 Ariyalur Ariyalur Arungal 2868 State Bank of India Ariyalur 19 Ariyalur Ariyalur Edayathankudi 2008 State Bank of India Ariyalur 20 Ariyalur -

The Following List of Candidates Are REJECTED for the Post of Reader, for the Reasons Mentioned in the Remarks Column
The following list of candidates are REJECTED for the post of Reader, for the reasons mentioned in the Remarks column. Reg. Name Address Date of Birth Sex Caste/ REMARKS RESULT No. Community RE00001 R.Sudhakar 5/38, Arunthathiyar Street, 05/10/1979 M SC Roster Quota not applicable Rejected Villupuram RE00002 S.Sathishkumar 2/4P, Govindswamy Street, 01/08/1991 M BC Roster Quota not applicable Rejected Chinnasekkadu, Manali, Chennai.68 RE00004 R. Aruntamizh David No. 118, East Street, Mokoor Village, 24/04/1995 M SC Roster Quota not applicable Rejected Sozhamandar Kudi Post, Sangarapuram TK, Villupuram. RE00005 D.Poonkodi 10C, Hospital Road, Roshanai, 15/05/1989 F SC Roster Quota not applicable Rejected Kuttakarai, Tindivanam Taluk, Villupuram District. RE00006 R.Vasantha No.211, School Street, Puthu Nagar, 01/05/1989 F SC Roster Quota not applicable Rejected Rajavallipuram, Tirunelveli-627 357. RE00009 K.Kannaiya No.211, School Street, Puthu Nagar, 25/05/1988 M SC Roster Quota not applicable Rejected Rajavallipuram, Tirunelveli-627 357. RE00010 S.Samuel Johnson No.15, Muthu Samay Nagar, 28/06/1980 M BC Roster Quota not applicable Rejected S.N.Chavadi, Cuddalore-1. RE00012 D.Rohini No.8, Ganesha flats, 19/05/1995 F SC Roster Quota not applicable Rejected Jamuna bai street, Sembiyam, Chennai-11 RE00013 M.Pushpavalli No.36, Thanam Nagar, 03/06/1989 F SC Roster Quota not applicable Rejected Thiruppapuliyur, Cuddalore-2 Pin-607 002. RE00014 R.Vinoth Main road, Maakuppam Post, 04/06/1991 M SC Roster Quota not applicable Rejected Thirukovilur Taluk, Villupuram District. RE00015 V.Jayasri 5/51, Throwpathi Amman Koil Street, 25/04/1981 F SC Roster Quota not applicable Rejected Melpattampakkam, Panruti Taluk, Cuddalore District. -

Cltn6july17.Pdf
Pradhan Mantri Gram Sadak Yojana Road List for Attachement With Sanction Letter Sanction Letter No :P-17024/24/2017-RC Sanction Date : 06/07/2017 State : Tamilnadu Sanction Year : 2017-2018 Batch : BATCH 1 Collaboration : Regular PMGSY Note : All Costs are in Lakhs and Lengths are in Kms. Sr.No. Core Network Name of Road / Category Road (Kms) / Carriage Stage CD Work MoRD Cost (Rs State Cost Total Cost Maint. Cost Habs (1000+, 500+, 250+, No. Bridge (N/U) Bridge (Mtrs) Way Const. (Nos) Lacs) (Including (Including (Rs Lacs) <250, Total) Length Width Additional Additional State Share) State Share) (Rs Lacs) (Rs Lacs) 1 2 3 4 5 6 7 8 9 10 11 12 13 Road Proposals District- Ariyalur Block- Andimadam 1 MRL01 MRL01- Upgarde 1.217 3.750 Complete 1 27.07 21.42 48.49 4.38 (2 , 0 , 0 , 0 , 2) Periyathukuruchi Nagambandal colony Road 2 MRL29 MRL29-Therkunatham Upgarde 2.699 3.750 Complete 2 36.63 30.25 66.89 9.72 (1 , 1 , 0 , 0 , 2) to Kattathur Road 3 MRL11 MRL11-Periyathathur - Upgarde 1.714 3.750 Complete 1 32.67 27.33 60.00 6.17 (1 , 2 , 0 , 0 , 3) Velichankudi to Keelaneduvai Road 4 MRL17 MRL17-Andimadam to Upgarde 3.900 3.750 Complete 2 62.65 51.40 114.05 14.04 (1 , 1 , 0 , 0 , 2) Pattanakuruchi - Melaneduvai Road 5 MRL12 MRL12-Periyathathur - Upgarde 3.275 3.750 Complete 1 45.19 37.59 82.78 11.79 (0 , 1 , 0 , 0 , 1) Keelaneduvai to Nettalakurichi road 6 MRL22 MRL22-Melur to Upgarde 2.400 3.750 Complete 3 38.73 31.27 69.99 8.64 (3 , 0 , 1 , 0 , 4) Devanur Kuvagam Road 7 MRL09 MRL09- Salakarai - Upgarde 5.385 3.750 Complete 2 72.67 63.90 136.57 -

Chapter – Iii Agro Climatic Zone Profile
CHAPTER – III AGRO CLIMATIC ZONE PROFILE This chapter portrays the Tamil Nadu economy and its environment. The features of the various Agro-climatic zones are presented in a detailed way to highlight the endowment of natural resources. This setting would help the project to corroborate with the findings and justify the same. Based on soil characteristics, rainfall distribution, irrigation pattern, cropping pattern and other ecological and social characteristics, the State Tamil Nadu has been classified into seven agro-climatic zones. The following are the seven agro-climatic zones of the State of Tamil Nadu. 1. Cauvery Delta zone 2. North Eastern zone 3. Western zone 4. North Western zone 5. High Altitude zone 6. Southern zone and 7. High Rainfall zone 1. Cauvery Delta Zone This zone includes Thanjavur district, Musiri, Tiruchirapalli, Lalgudi, Thuraiyur and Kulithalai taluks of Tiruchirapalli district, Aranthangi taluk of Pudukottai district and Chidambaram and Kattumannarkoil taluks of Cuddalore and Villupuram district. Total area of the zone is 24,943 sq.km. in which 60.2 per cent of the area i.e., 15,00,680 hectares are under cultivation. And 50.1 per cent of total area of cultivation i.e., 7,51,302 19 hectares is the irrigated area. This zone receives an annual normal rainfall of 956.3 mm. It covers the rivers ofCauvery, Vennaru, Kudamuruti, Paminiar, Arasalar and Kollidam. The major dams utilized by this zone are Mettur and Bhavanisagar. Canal irrigation, well irrigation and lake irrigation are under practice. The major crops are paddy, sugarcane, cotton, groundnut, sunflower, banana and ginger. Thanjavur district, which is known as “Rice Bowl” of Tamilnadu, comes under this zone. -

Tamil Nadu Public Service Commission Bulletin
© [Regd. No. TN/CCN-466/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 196/2009 2018 [Price: Rs. 145.60 Paise. TAMIL NADU PUBLIC SERVICE COMMISSION BULLETIN No. 7] CHENNAI, FRIDAY, MARCH 16, 2018 Panguni 2, Hevilambi, Thiruvalluvar Aandu-2049 CONTENTS DEPARTMENTAL TESTS—RESULTS, DECEMBER 2017 NAME OF THE TESTS AND CODE NUMBERS Pages Pages The Tamil Nadu Government office Manual Departmental Test for Junior Assistants In Test (Without Books & With Books) the office of the Administrator - General (Test Code No. 172) 552-624 and official Trustee- Second Paper (Without Books) (Test Code No. 062) 705-706 the Account Test for Executive officers (Without Books& With Books) (Test Code No. 152) 625-693 Local Fund Audit Department Test - Commercial Book - Keeping (Without Books) Survey Departmental Test - Field Surveyor’s (Test Code No. 064) 706-712 Test - Paper -Ii (Without Books) (Test Code No. 032) 694-698 Fisheries Departmental Test - Ii Part - C - Fisheries Technology (Without Books) Fisheries Departmental Test - Ii Part - B - (Test Code No. 067) 712 Inland Fisheries (Without Books) (Test Code No. 060) 698 Forest Department Test - forest Law and forest Revenue (Without Books) Fisheries Departmental Test - Ii Part - (Test Code No. 073) 713-716 A - Marine Fisheries (Without Books) (Test Code No. 054) 699 Departmental Test for Audit Superintendents of Highways Department - Third Paper Departmental Test for Audit Superintendents (Constitution of India) (Without Books) of Highways Department - First Paper (Test Code No. 030) 717 (Precis and Draft) (Without Books) (Test Code No. 020) 699 The Account Test for Public Works Department officers and Subordinates - Part - I (Without Departmental Test for the officers of Books & With Books) (Test Code No. -
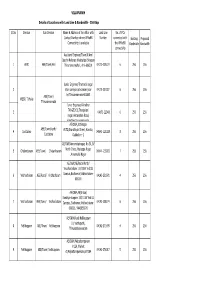
VILLUPURAM Sl.No Division Sub-Division Name & Address Of
VILLUPURAM Details of Locations with Land Line & Bandwidth - 256 Kbps Sl.No Division Sub-Division Name & Address of the office with Land Line No. of PCs Contact Number where VPNoBB Number connected with Existing Proposed Connectivity is available the VPNoBB Bandwidth Bandwidth connectivity Assistant Engineer/Town/N/Arni Opp to Nellarasi Viyabarigal Snagam 1 ARNI AEE/Town/Arni Thirumana mahal , Arni-606301 04173-225224 6 256 256 Junior Engineer/Thamarai nagar 2 Visiri samiyar ashramam(near 04175-237387 6 256 256 AEE/Town/ by)Tiruvannamalai-606603 WEST/ T.Malai Thiruvannamalai Junior Engineer/Kilnathur 3 TANGEDCO,Thenpalani 04175-223481 6 256 256 nagar,Vettavalam Road, kilnathur,Tiruvannamalai. AE/O&M,Jothinagar AEE/Town/North/ 4 Cuddalore #172,Bharathiyar Street, Kondur, 04142- 225229 8 256 256 Cuddalore Cuddalore -2 AE/O&M,Annamalainagar, No.50, IV 5 Chidambaram AEE/Town/ Chidambaram North Cross, Mariappa Nagar 04144- 239322 7 256 256 ,Annamalai Nagar AE/O&M,AE/Rural/North/ Virudhachalam 110/11KV Vrd SS 6 Vridhachalam AEE/Rural/ Vridhachalam Campus,Budhamur,Vridhachalam- 04143-231971 4 256 256 606001 AE/O&M,AE/Urban/ Kandiyankuppam 110/11KV Vrd SS 7 Vridhachalam AEE/Town/ Vridhachalam Campus, Budhamur,Vridhachalam- 04143-238274 6 256 256 606001. 9445856076 AE/O&M,Rural Nellikuppam 10, Vazhapattu, 8 Nellikuppam AEE/Town/ Nellikuppam 04142-271699 4 256 256 Thirukandeeswaram AE/O&M,Melpattampakam #12A ,Market 9 Nellikuppam AEE/Town/ Nellikuppam st,Melpattampakkam,607104 04142-276017 5 256 256 Assistant Engineer/Town I/ Villupuram, 110/11KV/SS -

Villupuram District
VILLUPURAM DISTRICT 1 VILLUPURAM DISTRICT 1. Introduction surrounded on East and South by Cuddalore District; the West by Salem and Dharmapuri i) Geographical location of the district districts and on the North by Thiruvannamalai and Kanchipuram districts. Villuppuram District lies between 11 38' 25" N and 12 20' 44" S: 78 15' 00" W ii) Administrative profile and 79 42' 55" E with an area of 7194 sq. km It was carved out from the South Arcot At present Villupuram district District on 30.09.1993 and was rechristened comprises of 1,490 revenue villages, 4 as Villuppuram District. The residual part revenue divisions, 9 administrative taluks 22 of the erstwhile South Arcot district was blocks, 15 town panchayat unions, 1,104 named as Cuddalore District. It is village panchayats and 3 municipalities. 2 iii) Meteorological information Types of soil in the district The district does not get heavy Red soil - Ulundurpet, Vanur, Gingee, rainfall with the exception of Marakanam Tindivanam and Vanur blocks, In Kandamangalam and Koliyaur blocks, the rainfall is moderate it is Black soil - Kallakurichi, Chinnasalem scarce in Kallakurichi and Sankarapuram. The total rainfall during the year 2002-03 Red sandy soil - Kanai , Thiruvennainallur was 617.4mm against 1030 mm of normal rainfall. The percentage of deviation was (-) ii) Agriculture and horticulture 38.9 mm. The average maximum and minimum temperatures for the district have The major crops grown in the district been 32.78 0 C in May and 24.08 0 C in are paddy, groundnut, sugarcane, cumbu, January respectively. gingelly and tapioca. Out of the total geographical area of 7.22 lakh ha the net 2. -

Villupuram Sl
PRIVATE SCHOOLS FEE DETERMINATION COMMITTEE CHENNAI-600 006 - FEES FIXED FOR THE YEAR 2013-2016 - DISTRICT: VILLUPURAM SL. SCHOOL HEARING SCHOOL NAME & ADDRESS YEAR LKG UKG I II III IV V VI VII VIII IX X XI XII NO. CODE DATE 2013 - 14 4500 4500 5690 5690 5690 5690 5690 6500 6500 6500 7000 7000 - - Kanchanandevi Matric School, 1 250001 18-03-13 2014 - 15 4950 4950 6259 6259 6259 6259 6259 7150 7150 7150 7700 7700 - - Alathur - 606 208. Villpuram. 2015 - 16 5445 5445 6885 6885 6885 6885 6885 7865 7865 7865 8470 8470 - - 2013 - 14 6000 6000 7000 7000 7000 7000 7000 8400 8400 8400 9500 9500 - - JAYAM MATRIC SCHOOL NEAR TALUK OFFICE 2 250002 18-3-13 2014 - 15 6600 6600 7700 7700 7700 7700 7700 9240 9240 9240 10450 10450 - - SANKARAPURAM VILLUPURAM 2015 - 16 7260 7260 8470 8470 8470 8470 8470 10164 10164 10164 11495 11495 - - 2013 - 14 5600 5600 6000 6000 6000 6000 6000 6200 6200 6200 7250 7250 - - CHANAKYA MATRIC SCHOOL PONDY ROAD 3 250003 6-5-13 2014 - 15 6160 6160 6600 6600 6600 6600 6600 6820 6820 6820 7975 7975 - - TINDIVANAM VILLUPURAM 2015 - 16 6776 6776 7260 7260 7260 7260 7260 7502 7502 7502 8773 8773 - - SRI MANAKULA VINAYAGAR 2013 - 14 5110 5110 6500 6500 6500 6500 6500 8000 8000 8000 9000 9000 - - MATRIC SCHOOL THIRUKOILUR HIGHWAY 4 250004 6-5-13 2014 - 15 5621 5621 7150 7150 7150 7150 7150 8800 8800 8800 9900 9900 - - METTUKUPPAM ELANDURAI VILLAGE VILLUPURAM 2015 - 16 6184 6184 7865 7865 7865 7865 7865 9680 9680 9680 10890 10890 - - 2013 - 14 6000 6000 7000 7000 7000 7000 7000 8200 8200 8200 - - -- SHANTHINAKETAN MATRIC SCHOOL 5 250005 6-5-13 2014 - 15 6600 6600 7700 7700 7700 7700 7700 9020 9020 9020 - - -- THIRUPPACHAVADIMEDU VILLUPURAM 2015 - 16 7260 7260 8470 8470 8470 8470 8470 9922 9922 9922 - - -- Om Guru Ganesasd 2013 - 14 5700 5700 6700 6700 6700 6700 6700 7775 7775 7775 9000 9000 - - Matriculation School No.1, Main Road, 6 250006 16-05-2013 2014 - 15 6270 6270 7370 7370 7370 7370 7370 8553 8553 8553 9900 9900 - - Subbiah Nagar, Kattu Edaiyar, Villupuram - 605 751. -

Viluppuram District
CENSUS OF INDIA 2011 TOTAL POPULATION AND POPULATION OF SCHEDULED CASTES AND SCHEDULED TRIBES FOR VILLAGE PANCHAYATS AND PANCHAYAT UNIONS VILUPPURAM DISTRICT DIRECTORATE OF CENSUS OPERATIONS TAMILNADU ABSTRACT VILUPPURAM DISTRICT No. of Total Sl. No. Panchayat Union Total Male Total Female Total SC SC Male SC Female Total ST ST Male ST Female Village Population 1 Thirukkoyilur 52 1,27,746 65,039 62,707 42,027 21,453 20,574 398 185 213 2 Mugaiyur 63 1,96,414 99,341 97,073 59,149 30,044 29,105 1,533 766 767 3 Thiruvennainallur 49 1,35,304 68,577 66,727 47,113 23,737 23,376 277 124 153 4 Tirunavalur 44 1,32,567 67,427 65,140 44,062 22,392 21,670 245 122 123 5 Ulundurpettai 53 1,50,054 75,113 74,941 49,007 24,691 24,316 236 118 118 6 Kanai 51 1,39,738 70,672 69,066 35,458 17,836 17,622 2,465 1,219 1,246 7 Koliyanur 43 1,19,915 59,930 59,985 37,444 18,498 18,946 601 312 289 8 Kandamangalam 45 1,45,181 72,400 72,781 51,755 25,583 26,172 219 110 109 9 Vikkiravandi 50 1,22,462 61,668 60,794 38,809 19,611 19,198 1,169 602 567 10 Olakkur 52 86,700 43,373 43,327 34,541 17,342 17,199 1,839 902 937 11 Mailam 47 1,17,439 58,872 58,567 42,231 21,223 21,008 1,624 811 813 12 Marakkanam 56 1,47,713 73,944 73,769 50,833 25,399 25,434 2,103 1,030 1,073 13 Vanur 65 1,64,696 83,132 81,564 58,365 29,388 28,977 2,513 1,257 1,256 14 Gingee 60 1,39,580 70,390 69,190 31,051 15,857 15,194 3,586 1,799 1,787 15 Vallam 66 1,09,270 55,335 53,935 29,588 15,095 14,493 2,201 1,108 1,093 16 Melmalayanur 55 1,41,155 70,621 70,534 27,261 13,597 13,664 2,375 1,175 1,200 17