1 Fluoride Distribution in Aquatic Environment in Vicinity of Aluminum Industry and Its Correlation 2 with Rainwater Chemistry and Weather Parameter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 | Page District Skill Development Plan:Sonbhadra
1 | P a g e District skill Development Plan:Sonbhadra “District Skill Development Plan” District- Sonbhadra Year-2020-21 District Project Management Team, Skill Development Mission, Sonbhadra, Uttar Pradesh Website: www.upsdm.gov.in, email: [email protected]/ [email protected] 2 | P a g e District skill Development Plan:Sonbhadra Contents As Is Scenario ......................................................................................................................................................................... 5 Executive Summary........................................................................................................................................................ 5 Overview ............................................................................................................................................................................. 6 District Composition ...................................................................................................................................................... 7 Target population: As per 2011 census ................................................................................................................ 7 Blockwise-Population ................................................................................................................................................... 7 District’s economic structure ........................................................................................................................................ -

Cop18 Prop. 51
Original language: English CoP18 Prop. 51 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________________ Eighteenth meeting of the Conference of the Parties Colombo (Sri Lanka), 23 May – 3 June 2019 CONSIDERATION OF PROPOSALS FOR AMENDMENT OF APPENDICES I AND II A. Proposal The proponents propose delisting of Dalbergia sissoo DC., from CITES Appendix II as it does not meet the listing criteria set out in Article II [(paragraphs 2(a)]of the Convention text or the criteria laid down in Annex 2(a) of Resolution Conf. 9.24 (Rev. CoP17). The species is widely distributed and in India it is found abundantly in wild and on cultivation as well. The species has very fast growth rate and capacity to become naturalized outside of its native range, even it is invasive in some parts of the world. The regulation of trade in the species is not necessary to avoid it becoming eligible for inclusion in Appendix I in the near future and the harvest of specimens from the wild is not reducing the wild population to a level at which its survival might be threatened by continued harvesting or other influences. B. Proponent Bangladesh, Bhutan, India and Nepal*: C. Supporting statement 1. Taxonomy 1.1 Class: Magnoliopsida 1.2 Order: Fabales 1.3 Familly: Fabaceae 1.4 Genus, species or subspecies, including author and year: DalbergiaL. f.1781 [1782] 1.5 Scientific synonyms: Amerimnon sissoo (Roxb. ex DC.) Kuntze 1.6 Common names: English: Indian rosewood, Himalaya raintree, Indian Dalbergia, Penny leaf tree, Sisso French: Spanish: 1.7 Code numbers: Not available 2. -

ANALYSIS of FOLDS from the CGGC ROCKS in SONBHADRA DISTRICT UTTAR PRADESH and THEIR TECTONIC and GEOMORPHIC IMPLICATIONS � � Vaibhava Srivastava and H
1 Journal of Scientific Research Vol. 56, 2012 : 1-18 Banaras Hindu University, Varanasi ISSN : 0447-9483 ANALYSIS OF FOLDS FROM THE CGGC ROCKS IN SONBHADRA DISTRICT UTTAR PRADESH AND THEIR TECTONIC AND GEOMORPHIC IMPLICATIONS Vaibhava Srivastava and H. B. Srivastava Centre of Advanced Study, Department of Geology Banaras Hindu University, Varanasi -221005 Abstract The Precambrian rocks exposed in the southern part of Sonbhadra district of Uttar Pradesh rocks form a part of Chhotanagpur plateau of the central part of India. Geologically it belongs to the Chhotanagpur Granite Gneiss Complex (CGGC) which has witnessed multiple tectonic deformations. From the analysis of the folds of this region four deformation phases have been identified for the CGGC rocks. The characteristic fold interference of dome and basin type has rendered the present day topography of the Chhotanagpur plateau which is different from nearby plateaux on other Precambrian rocks in central India. Introduction The structural features are the manifestations of the deforming forces that have acted on the rock bodies since their formation. Each rock type possesses different mechanical and physical properties which vary with conditions under which it deforms. Therefore, diverse varieties of structures are displayed by the rocks which constitute the earth's crust. The systematic study of these structures i.e. the structural analysis reveals the deformational histories of the rock bodies in different parts of the crust. Folds are those structures which develop during the ductile deformation of the layered rocks in response to the tectonic forces. Therefore the systematic studies and analysis can reveals many aspects of the tectonic events that the rocks has undergone. -

Inspirations of Traditional Hindu Temple Architecture in the Birla Mandir in Renukoot
AAgnieszkagnieszka SStaszczyktaszczyk Jagiellonian University in Kraków Polish Institute of World Art Studies INSPIRATIONS OF TRADITIONAL HINDU TEMPLE ARCHITECTURE IN THE BIRLA MANDIR IN RENUKOOT he Renukoot (Reṇukūṭ) Birla mandir, dedicated to Śiva in the aspect of Reṇukeśvara Mahādeva, was founded in 1972 by the Birla (Biṛlā) Tfamily. It is located in one of the Birlas’ industrial towns — Renukoot, in the south-eastern part of Uttar Pradeś where HINDALCO Industries Limited (the aluminium factory) has operated since 1962. The region started attracting investors and industrialist after a dam was constructed on the river Rihand1). It was inaugurated in 1960 by the then prime minister of India – Jawaharlal Nehru. He tried to encourage every guest and businessman who came there, among them Ghanshyamdas Birla, to invest in the region. Indeed, the Birlas decided to locate one of their main enterprises here — the Hindalco (Hin- dustan Aluminium Corporation) factory. It may be assumed that the temple was planned to serve as the main shrine for the Hindu community living and working in this area. At the same time, it is one of the attractions for visitors to the region. It must, however, be mentioned that if at all we consider it as a kind of ‘tourist’ place, it is only so for local (Indian) visitors2), since it is located beyond the main tourist routes and is rather difficult to reach. The 1) Kudaisya (2003: 334–335). 2) We may find few references to the temple on some websites informing about this area and the attractions one may consider while visiting it, e.g. -

Sociedade E Natureza V.23
Geografi a, pobreza e desenvolvimento: um debate interdisciplinar Anand Prasad Mishra GEOGRAPHY, POVERTY AND DEVELOPMENT: AN INTERDISCIPLINARY DEBATE Geografi a, pobreza e desenvolvimento: um debate interdisciplinar Anand Prasad Mishra Associate professor, department of geography, Faculty of science Banaras Hindu University. Varanasi 221005 – Uttar Pradesh – India [email protected] [email protected] Artigo recebido para publicação em 20/02/2011e aceito para publicação em 20/06/2011 ABSTRACT: An emerging feature of contemporary development studies in India is the deployment of an interdisci- plinary approach involving geographical location, level of poverty, nature of development and planning etc. The prevalence of poverty in a specifi c geographical location represents the evolving pattern of deprivation under a particular mode of production. The historicity of poverty in a geographical space needs an independent enquiry and identifi cation of different production systems which are responsible for the problem of deprivation through multiple routes. The present paper is an attempt to initiate a debate on the issue of poverty, especially in a tribal region, through a multi-dimensional perspective, i.e. interrelation between geography, poverty, development and planning. The paper identifi es one of the most poverty-stricken regions of India for a detailed discussion of the various casual factors which are apparently responsible for the poverty of that region. The paper also tries to explore the historical background of poverty in the study area (Babhani Block of Sonbhadra U. P.). Keywords: Poverty. Deprivation. Geography of poverty. Historical routes of poverty. RESUMO: Uma característica emergente dos estudos de desenvolvimento na Índia contemporânea é a implantação de uma abordagem interdisciplinar envolvendo localização geográfi ca, nível de pobreza, a natureza do desenvolvimento e planejamento, entre outros. -

Mercury Pollution in Sonbhadra District of Uttar Pradesh and Its Health Impacts
CSE Study: Mercury Pollution in Sonbhadra District of Uttar Pradesh and its Health Impacts Mercury Pollution in Sonbhadra District of Uttar Pradesh and its Health Impacts INVESTIGATORS Mr. Ramakant Sahu Ms. Poornima Saxena Dr. Sapna Johnson Ms. Soundaram Ramanathan Ms. Sugandh Juneja ADVISORS Prof. (Dr.) H. B. Mathur Prof. (Dr.) H. C. Agarwal October 2012 CENTRE FOR SCIENCE AND ENVIRONMENT 41, TUGHLAKABAD INSTITUTIONAL AREA, NEW DELHI –110062 TEL: 91-11-2995 6110/5124/6394/6399 FAX: 91-11-2995 5879 EMAIL: [email protected] WEBSITE: www.cseindia.org POLLUTION MONITORING LABORATORY CORE-6A, FOURTH FLOOR, INDIA HABITAT CENTRE LODHI ROAD, NEW DELHI – 110003 1 CSE Study: Mercury Pollution in Sonbhadra District of Uttar Pradesh and its Health Impacts CONTENTS 1. Pollution Monitoring Laboratory of CSE 3 2. The Study 3 3. Introduction 4 4. Review of Literature 10 5. Health Effects 16 6. Objectives of the Study 19 7. Materials and Methods 19 8. Results and Discussion 26 9. Conclusions 36 10. Recommendations 38 11. Annexures 46-47 Annexure 1: Village Wise Distribution of Mercury in Human Blood, Hair and Nail in Singrauli Annexure 2: Concentration of Mercury in Human Blood, Hair and Nail in Singrauli on Gender basis 2 CSE Study: Mercury Pollution in Sonbhadra District of Uttar Pradesh and its Health Impacts 1. POLLUTION MONITORING LABORATORY OF CSE The Centre for Science and Environment (CSE), a non-governmental organization based in New Delhi, has set up the Pollution Monitoring Laboratory (PML) to monitor environmental pollution. PML is an ISO 9001:2008 certified laboratory accredited by SWISO, CH-5610, Wohlen, Switzerland, conducting Pollution Monitoring and Scientific Studies on Environmental Samples. -
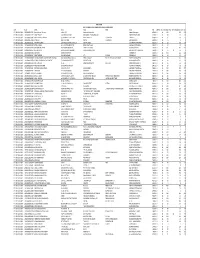
SHRTRF-1314I-Alankit Mail Dated 17.3.2021.Xlsx
NTPC LIMITED LIST OF SHARES TO BE TRANSFERRED TO IEPF IN APRIL, 2021 YEAR FOLIO NAME ADD1 ADD2 ADD3 ADD4 PIN UNPAID CNT SHR1314I PREV_IEPF SHR1232021 SHR_TRF INT. DIV. 2013‐2014 1201060000130231 Ramesh Kumar Sharma Laltanki,C/O Ramkumar Ramesh Kr Raigarh Chhatisgarh 496001 U 14 100 120 100 INT. DIV. 2013‐2014 1201060000213527 SUMIT THAKUR I‐18, SEALTAX COLONY KHAMARDIH, SHANKAR NAGAR RAIPUR CHHATISGARH 492001 U 14 30 15 15 INT. DIV. 2013‐2014 1201060000349183 MAHENDRA KESHARBANI S/O RATAN LAL KESHARBANI BANDHWA PARA PURANI BASTI RAIPUR C G 492001 U 14 25 30 25 INT. DIV. 2013‐2014 1201060001479852 PRABHASH . MADHAVI RAMS, NADAL PO EDAKKAD KANNUR KERALA 670001 U 14 5 1 1 INT. DIV. 2013‐2014 1201060001587351 RAM NIVAS SINGH ELECTRICAL EXCUTIVE ENGINEER CENTRAL STORE DALTONGANJ JHARKHAND 822101 U 14 50 90 50 INT. DIV. 2013‐2014 1201060400061765 RITESH KUMAR MOH. MEHTAN JWALAPUR NEAR SANGITA HALL HARIDWAR UTTRANCHAL 249407 U 14 16 19 16 INT. DIV. 2013‐2014 1201060500037048 RAM KISHORE MEENA VILLAGE ROHADE KALAN TEH & DIST ‐ DAUSA DAUSA RAJASTHAN 303303 U 14 161 193 161 INT. DIV. 2013‐2014 1201060500283521 DEEPAK GUPTA AGRASEN COLONY BBEHIND OSWAL MARRIGE HOME GANGAPUR CITY RAJASTHAN 322201 U 14 75 70 70 5 INT. DIV. 2013‐2014 1201060600035484 SUBAS ROI 4/909 VIKAS NAGAR LUCKNOW U.P. 226022 U 14 214 256 214 INT. DIV. 2013‐2014 1201060600065397 SUMIT RASTOGI BANK OF BAROUDA 970, NIYAWAN FAIZABAD U.P. 224001 U 14 10 12 10 INT. DIV. 2013‐2014 1201070000095866 SANTBAKSHSINGH SAHADEOSINGH CHAUHAN MANORAMA NIWAS, 102/14‐A PENSION NAGAR POLICE LINE TAKLI, KATOL ROAD NAGPUR MAHARASHTRA 440013 U 14 85 102 85 INT. -
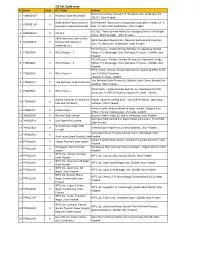
DC List Cycle Wise Sr
DC list Cycle wise Sr. NoSector Cycle DC's Name Address Hindalco Industries Limited,P. O. Renukoot, Dist- Sonbhadra, Pin- 1 ALM0009UP II Hindalco Industries Limited 231217, Uttar Pradesh Dalla Cement Factory (Unit of Dalla Cement Factory (Unit of Jaiprakash Associates Limited) ,S.H. 5 2 CMT0051UP II Jaiprakash Associates Limited) kota P.O. Dalla Distt.Sonebhadra , Uttar Pradesh ACC Ltd., Tikaria Cement Works,PO- Gauriganj District -Chhatrapati 3 CMT0083UP II ACC Ltd. Shahuji Maharaj Nagar, , Uttar Pradesh ABCIL Renukoot Chemical Div. ABCIL Renukoot Chemical Div. (Kanoria Chemicals and Industries 4 CNA0012UP II (Kanoria Chemicals and Ltd.), P.O. Renukoot , Sonebhadar, Uttar Pradesh Industries Ltd.) IFFCO Phulpur - I,Indian Farmers Fertilizer Co- Operative Limited , 5 FTZ0013UP II IFFCO Phulpur - I Phulpur P.O Ghiyanagar, Dist. Allahabad ,Pin Code – 212404, Uttar Pradesh IFFCO Phulpur - II,Indian Farmers Fertilizer Co- Operative Limited , 6 FTZ0018UP II IFFCO Phulpur - II Phulpur P.O Ghiyanagar, Dist. Allahabad ,Pin Code – 212404, Uttar Pradesh IFFCO, Aonla - II,Indian Farmers Fertilizer Co- Operative IFFCO Aonla 7 FTZ0022UP II IFFCO Aonla - II unit, P.O IFFCO Township , Bareilly Pin Code - 243403 Yara Fertilisers India Private Ltd., Babrala, Indira Dham, Babrala Dist- 8 FTZ0024UP II Yara Fertilisers India Private Ltd. Sambhal, Uttar Pradesh IFFCO Aonla - I,Indian Farmers Fertilizer Co- Operative Ltd IFFCO 9 FTZ0025UP II IFFCO Aonla - I Aonla unit, P.O IFFCO Township , Bareilly Pin Code – 243403 Grasim Industries Limited (Unit - Grasim Industries Limited (Unit - Indo Gulf Fertilisers) , Jagdishpur, 10 FTZ0026UP II Indo Gulf Fertilisers) , Sultanpur, Uttar Pradesh Kribhco Shyam, Kribhco Shyam Fertilizer Limited , Village & Post 11 FTZ0027UP II Kribhco Shyam Office :, Piprola, Shahjahanpur , Pin Code - 242001 12 INS0051UP II Bhushan Steel Limited. -

Stone Crushers Sonebhadra District NGT Order.Pdf
BEFORE THE NATIONAL GREEN TRIBUNAL PRINCIPAL BENCH NEW DELHI ********** ORIGINAL APPLICATION NO. 482 OF 2015 (M.A. NO. 574/2016) IN THE MATTER OF: Chaudhary Yashwant Singh Son of Late Sri Ratiram Singh Aged about 54 years R/o. Vikas Nagar, Ward No. 11 Robertsganj, Sonebhadra …..Applicant Versus 1. State of Uttar Pradesh Through the Chief Secretary Government of U.P. at Lucknow U.P. 2. Member Secretary U.P. Pollution Control Board TC-12, Vibhuti Khand, Gomti Nagar Lucknow, U.P. 3. Managing Director U.P. Rajkiya Nirman Nigam Ltd. Vibhuti Khand, Gomti Nagar, Lucknow 4. District Magistrate Sonebhadra U.P. 5. Member Secretary, Ministry of Environment Forest and Climate Change, Govt. of India New Delhi 6. State of Uttar Pradesh Through its Chief Forest Conservator Uttar Pradesh, Lucknow, U.P. Pin Code No. 226001 7. State of U.P. Through its Principal Secretary, Forest Uttar Pradesh, Lucknow, U.P. Pin Code No. 226001 1 8. Principal Secretary Mines and Mineral Uttar Pradesh, Lucknow, U.P. Pin Code No. 226001 9. District Mining Officer District Sonebhadra, U.P. Pin Code No. 231216 10. JAY PEE Industries Ltd. (J.P. Cements, Dala/Churk) Dala District Sonebhadra, U.P. Pin Code No. 231207 11. HINDALCO Industries Ltd. (Renusagar Power Division Renusagar) District Sonebhadra, U.P. Pin Code No. 231218 12. LANCO Anpara Power Ltd. Gate No. 3 Anpara, Post Anpara District Sonebhadra, U.P. Pin Code No. 231225 13. CEO UPSHA (Uttar Pradesh State Highway Authority) Pin Code No. 226001 14. Project Manager Chetak Construction Co./A.C.P. Tollyways Pvt. -

Varanasi-Shaktinagar Section of NH-5A in Mirzapur, Sonbhadra, Obra, Renukut Forest Divisions and Kaimur Wild Life Sanctuary in the State of Uttar Pradesh – Regarding
F. No. 8-86/2012-FC Diversion of 129.251 ha of forest land in favour of Uttar Pradesh State Highway Authority (UPSHA) for widening/ upgradation of Varanasi-Shaktinagar section of NH-5A in Mirzapur, Sonbhadra, Obra, Renukut Forest Divisions and Kaimur Wild life Sanctuary in the State of Uttar Pradesh – regarding. 1. The State Government of Uttar Pradesh vide its letter no. 2636/14-2-2012-800(70)/2012 dated 12.10.2012 has submitted the above mentioned diversion proposal for seeking prior approval of the Central Government in accordance with Section – 2 of the Forest (Conservation) Act, 1980. 2. The proposed road envisages widening and upgrdation of existing State Highway to four lane with paved shoulders starting from Naray7anpur (in Mirzapur District) to Sonbhadra, Dala and upto Shaktinagar. The present proposal has been proposed keeping in view increased traffic and to ensure traffic safety. As per the State Government order dated 14.05.2010, a paln of Rs. 1211.96 has been prepared to develop the Highway on PPP basis. 3. The Regional Office, Lucknow of this Ministry conducted the site Inspection of the proposed area and submitted report to this Ministry vide letter dated 3rd January, 2013. The regional Office has recommended the proposal subject to fulfilment of certain conditions (Pg 166/c). 4. The facts related to the proposal as contained in the State Government’s letter dated 12.10.2012 are given as under: A. FACT SHEET 1. Name of the Proposal Diversion of 129.251 ha of forest land for widening/upgradation of NH-5A (Varanasi-Shaktinagar Section) from Km 0.00 to 117.650 in Mirzapur, Sonbhadra, Obra, Renukoot forest Divisions and kaimur Wildlife Sanctuary in the state of Uttar Pradesh in favour of U. -

Cgst & Central Excise Commissionerate, Varanasi
CGST & CENTRAL EXCISE COMMISSIONERATE, VARANASI Address: 9, Maqbool Alam Road, near Zila Kutchehari, Varanasi-221002. Phone:0542-2502864 E mail:[email protected] Jurisdiction The territorial jurisdiction of CGST and Central Excise Commissionerate Varanasi, extends to Districts of Varanasi, Chandauli, Ghazipur, Azamgarh, Ballia, Mau, Gorakhpur, Kushinagar, Maharajganj, Deoria, Sant Kabir Nagar, Siddharth Nagar, Mirzapur and Sonebhadra of the state of Uttar Pradesh. The CGST & Central Excise Commissionerate Varanasi comprises of following Divisions headed by Deputy/ Assistant Commissioners. 1. Division: Varanasi 2. Division: Azamgarh 3. Division: Gorakhpur-I 4. Division: Gorakhpur-II 5. Division: Mirzapur Jurisdiction of Divisions & Ranges: NAME OF DIVISION JURISDICTION NAME OF RANGE JURISDICTION OF RANGE DIVISION Varanasi Area covering North Varanasi /Varanasi PS – Jaitpura, Adampur, Division Ranges of North, Kotwali & Cantt (South Of East, South, West River Varuna) and Sarnath under East Varanasi/Varanasi PS – Dasashwamedh, Luxa, Varanasi district and Sigra, Chetganj, Chowk. entire Chandauli South Varanasi/Varanasi PS- Lanka, Manduadih, district. Bhelupur, Ramnagar West Varanasi/Varanasi PS-Lohta, Mirzamurad, Rohania, Jansa Sarnath/Varanasi/Varanasi PS-Cantt(North Of River Varuna), Sarnath, Phoolpur, Badagaw, Chaubeypur & Shivpur Chandauli/Chandauli Whole of District Chandauli Azamgarh Territory under the Ghazipur/Ghazipur Whole of District Ghazipur Division Districts of Ghazipur, Azamgarh/Azamgarh Whole of District Azamgarh Azamgarh, Ballia & Ballia/Ballia Whole of District Ballia Mau. Mau/Mau Whole of District Mau Gorakhpur-I Area covering Area of P.S Gorakhnath, Division Ranges of Urban-I, P.S Shahpur, P.S Gularia, Urban-II and Urban – I/Gorakhpur P.S Chilwatal and Tehsil districts Kushinagar, Campierganj of District Maharajganj & Gorakhpur Deoria. -

U.P.S.R.T.C. Time Table Index Janrath Buses (Up Dated Up
U.P.S.R.T.C. TIME TABLE INDEX JANRATH BUSES (UP DATED UP TO 15-05-2019) PRAYAGRAJ GHAZIABAD AZAMGARH GORAKHPU SHAHARAN SHAHARAN LUCKNOW VARANASI ALIGARH ETAWAH MEERUT KANPUR JHANSI AGRA PUR S.No. Route Services Buses Trip R 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 1 LUCKNOW-PRAYAGRAJ 19 20 38 8 12 2 PRAYAGRAJ TO JAUNPUR-AZAMGARH-GORAKHPUR 13 13 26 7 6 3 LUCKNOW-GORAKHPUR 34 34 68 9 11 14 4 VARANASI-AZAMGARH-GORAKHAPUR 14 14 28 9 5 5 LUCKNOW-VARANASI VIA SULTANPUR 6 6 12 1 5 6 VARANASI- PRAYAGRAJ- KANPUR 18 18 36 14 4 7 AGRA-LUCKNOW EXP-WAY 2 2 4 1 1 8 AGRA-KANPUR 4 4 8 2 2 9 GORAKHPUR-LUCKNOW-ANANDVIHAR 4 12 8 12 10 AGRA-MEERUT 2 2 4 2 11 PRAYAGRAJ TO KANPUR 7 7 14 7 12 PRAYAGRAJ-SHAKTINAGAR 2 2 4 2 13 PRAYAGRAJ TO BANDA 1 1 2 1 14 PRAYAGRAJ TO BASTI 1 1 2 1 15 MEERUT-DELHI 1 1 2 1 16 MEERUT-SHAMLI-LUCKNOW 1 2 2 2 17 JHASI-KANPUR-LUCKNOW 4 4 8 4 18 MAU-CHIRAIYAKOT-FAIZABAD-KANPUR 1 2 2 2 19 VARANASI-KANPUR-MATHURA 1 2 2 2 20 VARANASI-SHAKTINAGAR-BAIDAN 6 6 12 6 21 VARANASI-SIGROULI 2 2 4 2 22 LUCKNOW-SULTANPUR-AZAMGARH 3 3 6 3 23 LUCKNOW-FAIZBAD-AZAMGARH 1 1 2 1 24 LUCKNOW-KARVI VIA FATEHPUR BANDA 2 2 4 2 25 LUCKNOW-MATHURA VIA AURAIYA ETAWA 2 4 4 4 26 LUCKNOW-RUPAIHDIA 6 6 12 4 2 27 LUCKNOW-DUMARIAGANJ-BADANI VIA GONDA 2 2 4 2 28 LUCKNOW-DELHI VIA BANGARMAU KANNAUJ 1 2 2 2 29 LUCKNOW-KANPUR 4 2 8 2 30 LUCKNOW-JAIPUR VIA KANPUR AGRA 1 2 2 2 31 LUCKNOW-KOTA VAI JHANSI 2 3 4 3 32 LUCKNOW-ALIGARH- VIA KANPUR 1 2 2 2 33 LUCKNOW-DUMARIAGANJ VIA GONDA 1 1 2 1 34 LUCKNOW-DELHI- VIA BAREELY 2 4 4 2 2 35 LUCKNOW-HARDOI 2 1 4 1 36 LUCKNOW-GAURIFANTA 1 1 2 1 37 LUCKNOW-RAMNAGER VIA BARELLLY 1 2 2 2 38 LUCKNOW-MAHOBA 1 1 2 1 39 LUCKNOW-BAHRICH 3 3 6 3 40 LUCKNOW-MIRZAPUR VIA ALLAHBAD 1 1 2 1 U.P.S.R.T.C.