Topics in Immunopathology 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Retrospective Approach to Evaluate Interferences in Immunoassay
Retrospective approach to evaluate interferences in immunoassay Jayesh Warade Laboratory Services, Meenakshi Mission Hospital and Research Center, Madurai, Tamilnadu, India INFO ABSTRACT Corresponding author: Background Jayesh Warade Laboratory Services Despite the increase in sensitivity and specificity of Meenakshi Mission Hospital immunoassay technique over years, analytical inter- and Research Center Madurai, Tamilnadu ference remains to be major area of concern. India E-mail: [email protected] The interfering substances are endogenous substances that are natural, polyreactive antibodies as heterophil- Key words: ic or auto antibodies, or human anti-animal antibodies heterophilic - antibody, immunoassay, interferences, cross reactive together with other unsuspected binding proteins that are unique to the individual. Interfering substances can interfere with the reaction between analyte and reagent antibodies in immunoassay resulting in false positive or negative values. This ultimately results in misinterpretation of patients reports and finally to wrong course of treatment. Objective In our study, we used a retrospective approach to find out the extent of interferences and type of interfer- ences in some cases during our routine practice. Method The immunoassay reports which were clinically not correlating were retrospectively evaluated after dis- cussion with the clinician. Over a period of six month a total of 42 samples were evaluated for interference for Page 224 eJIFCC2017Vol28No3pp224-232 Jayesh Warade Retrospective approach to evaluate interferences in immunoassay different immunoassay parameters such as Beta for prior extraction, immunoassays may lack ad- HCG, Estradiol, CA 125, AFP, prolactin, Hepatitis equate specificity and accuracy (1). B Surface antigen (HbSAg) and troponin I. The Developing immunoassays for the quantifica- samples were treated with commercially avail- tion of an analyte in a buffer solution has its able antibody blocking agents and were reana- own challenges, nonetheless quantification of lyzed. -

Clinical Pathology, Immunopathology and Advanced Vaccine Technology in Bovine Theileriosis: a Review
pathogens Review Clinical Pathology, Immunopathology and Advanced Vaccine Technology in Bovine Theileriosis: A Review Onyinyechukwu Ada Agina 1,2,* , Mohd Rosly Shaari 3, Nur Mahiza Md Isa 1, Mokrish Ajat 4, Mohd Zamri-Saad 5 and Hazilawati Hamzah 1,* 1 Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang 43400, Malaysia; [email protected] 2 Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, University of Nigeria Nsukka, Nsukka 410001, Nigeria 3 Animal Science Research Centre, Malaysian Agricultural Research and Development Institute, Headquarters, Serdang 43400, Malaysia; [email protected] 4 Department of Veterinary Pre-clinical sciences, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang 43400, Malaysia; [email protected] 5 Research Centre for Ruminant Diseases, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang 43400, Malaysia; [email protected] * Correspondence: [email protected] (O.A.A.); [email protected] (H.H.); Tel.: +60-11-352-01215 (O.A.A.); +60-19-284-6897 (H.H.) Received: 2 May 2020; Accepted: 16 July 2020; Published: 25 August 2020 Abstract: Theileriosis is a blood piroplasmic disease that adversely affects the livestock industry, especially in tropical and sub-tropical countries. It is caused by haemoprotozoan of the Theileria genus, transmitted by hard ticks and which possesses a complex life cycle. The clinical course of the disease ranges from benign to lethal, but subclinical infections can occur depending on the infecting Theileria species. The main clinical and clinicopathological manifestations of acute disease include fever, lymphadenopathy, anorexia and severe loss of condition, conjunctivitis, and pale mucous membranes that are associated with Theileria-induced immune-mediated haemolytic anaemia and/or non-regenerative anaemia. -
Case Reports & Imaging Journal False Negative Cryptococcus Latex
Annangi S, et al. J Case Repo Imag 2017 1: 002 Case Reports & Imaging Journal Case Report False Negative Cryptococcus Latex Agglutination Test Despite Correcting for Conventional Reasons: A Case Report Srinadh Annangi1, Snigdha Nutalapati1 and Mesfin Fransua2* 1Department of Internal Medicine, Morehouse School of Medicine, Atlanta, GA, USA 2Department of Internal Medicine, Division of Infectious Diseases, Morehouse School of Medicine, Atlanta, GA, USA *Corresponding author: Mesfin Fransua, Department of Internal Abstract Medicine, Division of Infectious Disease, Morehouse School of Med- Cryptococcus meningitis needs high index of suspicion given its icine, 720, Westview Dr, Atlanta, GA, USA, Tel: +1 4047561366; subtle presentation. Though the gold standard for diagnosis is cul- E-mail: [email protected] ture of cerebrospinal fluid, given the delay in culture results, treat- ment is usually initiated with positive latex agglutination test or Indian Received Date: November 28, 2016 ink staining. A 50 years old male with HIV/AIDS (Human immuno- deficiency virus infection/ acquired immune deficiency syndrome) Accepted Date: March 28, 2017 non compliant with antiretroviral therapy presented complaining of throbbing non radiating occipital headache. Physical examination Published Date: April 12, 2017 is unremarkable and computerized tomography of head showed no evidence of mass lesions or raised intracranial pressure. Cryptococ- Citation: Annangi S, Nutalapati S, Fransua M (2017) False Negative cal meningitis is suspected especially given CD4 count of 19 cells/ Cryptococcus Latex Agglutination Test Despite Correcting for Conven- mcL. Cerebrospinal Fluid (CSF) Indian ink stains and Cryptococcus tional Reasons: A Case Report. J Case Repo Imag 1: 002. antigen latex agglutination test from pronase treated CSF sample was negative. -
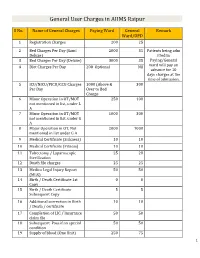
General User Charges in AIIMS Raipur
General User Charges in AIIMS Raipur S No. Name of General Charges Paying Ward General Remark Ward/OPD 1 Registration Charges 200 25 2 Bed Charges Per Day (Sami 2000 35 Patients being adm Deluxe) itted in 3 Bed Charges Per Day (Deluxe) 3000 35 Paying/General 4 Diet Charges Per Day 200 Optional Nil ward will pay an advance for 10 days charges at the time of admission. 5 ICU/NICU/PICU/CCU Charges 1000 (Above & 300 Per Day Over to Bed Charge 6 Minor Operation in OT/MOT 250 100 not mentioned in list, under L A 7 Minor Operation in OT/MOT 1000 300 not mentioned in list, under G A 8 Major Operation in OT, Not 2000 1000 mentioned in list under G A 9 Medical Certificate (Sickness) 10 10 10 Medical Certificate (Fitness) 10 10 11 Tubectomy / Laparoscopic 25 20 Sterilization 12 Death file charges 25 25 13 Medico Legal Injury Report 50 50 (MLR) 14 Birth / Death Certificate 1st 0 0 Copy 15 Birth / Death Certificate 5 5 Subsequent Copy 16 Additional correction in Birth 10 10 / Death / certificate 17 Completion of LIC / Insurance 50 50 claim file 18 Subsequent Pass if on special 50 50 condition 19 Supply of blood (One Unit) 250 75 1 20 Medical Board Certificate 500 500 On Special Case User Charges for Investigations in AIIMS Raipur S No. Name of Investigations Paying General Remark Ward Ward/OPD Anaesthsia 1 ABG 75 50 2 ABG ALONGWITH 150 100 ELECTROLYTES(NA+,K+)(Na,K) 3 ONLY ELECTROLYTES(Na+,K+,Cl,Ca+) 75 50 4 ONLY CALCIUM 50 25 5 GLUCOSE 25 20 6 LACTATE 25 20 7 UREA. -

Practice Parameter for the Diagnosis and Management of Primary Immunodeficiency
Practice parameter Practice parameter for the diagnosis and management of primary immunodeficiency Francisco A. Bonilla, MD, PhD, David A. Khan, MD, Zuhair K. Ballas, MD, Javier Chinen, MD, PhD, Michael M. Frank, MD, Joyce T. Hsu, MD, Michael Keller, MD, Lisa J. Kobrynski, MD, Hirsh D. Komarow, MD, Bruce Mazer, MD, Robert P. Nelson, Jr, MD, Jordan S. Orange, MD, PhD, John M. Routes, MD, William T. Shearer, MD, PhD, Ricardo U. Sorensen, MD, James W. Verbsky, MD, PhD, David I. Bernstein, MD, Joann Blessing-Moore, MD, David Lang, MD, Richard A. Nicklas, MD, John Oppenheimer, MD, Jay M. Portnoy, MD, Christopher R. Randolph, MD, Diane Schuller, MD, Sheldon L. Spector, MD, Stephen Tilles, MD, Dana Wallace, MD Chief Editor: Francisco A. Bonilla, MD, PhD Co-Editor: David A. Khan, MD Members of the Joint Task Force on Practice Parameters: David I. Bernstein, MD, Joann Blessing-Moore, MD, David Khan, MD, David Lang, MD, Richard A. Nicklas, MD, John Oppenheimer, MD, Jay M. Portnoy, MD, Christopher R. Randolph, MD, Diane Schuller, MD, Sheldon L. Spector, MD, Stephen Tilles, MD, Dana Wallace, MD Primary Immunodeficiency Workgroup: Chairman: Francisco A. Bonilla, MD, PhD Members: Zuhair K. Ballas, MD, Javier Chinen, MD, PhD, Michael M. Frank, MD, Joyce T. Hsu, MD, Michael Keller, MD, Lisa J. Kobrynski, MD, Hirsh D. Komarow, MD, Bruce Mazer, MD, Robert P. Nelson, Jr, MD, Jordan S. Orange, MD, PhD, John M. Routes, MD, William T. Shearer, MD, PhD, Ricardo U. Sorensen, MD, James W. Verbsky, MD, PhD GlaxoSmithKline, Merck, and Aerocrine; has received payment for lectures from Genentech/ These parameters were developed by the Joint Task Force on Practice Parameters, representing Novartis, GlaxoSmithKline, and Merck; and has received research support from Genentech/ the American Academy of Allergy, Asthma & Immunology; the American College of Novartis and Merck. -

Microbiology and Immunology
College of Medicine MI Microbiology and Immunology MI 494G IMMUNOBIOLOGY. (3) A survey of theories and mechanisms of immunity, including: nature of antigens and antibodies, antigen-antibody reactions, immunocompetent cells, immunogenetics, allergic reactions, tumor immunology and transplantation immunology. Prereq: BCH 401G (may be taken concurrently) and BIO 208 or BIO 308 or consent of instructor. (Same as BIO 494G.) MI 590 CELLULAR AND MOLECULAR PHYSIOLOGY. (4) This course will focus on the cellular and molecular physiology of inter-and intracellular communication. In particular, it will provide an overview of established and emerging intracellular signaling mechanisms which utilize i) cyclic nucleotides (cAMP; cGMP), ii) calcium (phosphatidylinositol metabolism: cyclic ADP-ribose), iii) transmembrane ion fluxes (voltage- and receptor-operated channels), iv) tyrosine kinases, and v) nuclear transcription factors. The material will be presented in a number of formats including didactic lecture and group discussions of selected readings. Prereq: PGY 412G, PGY 502 or consent of instructor. (Same as PGY 590.) MI 595 IMMUNOBIOLOGY LABORATORY. (2) Laboratory in immunology and serology. Preparation, standardization, and uses of biological products; serology. Laboratory; four hours. Prereq: BIO/MI 494G or concurrently; or consent of instructor. (Same as BIO 595.) MI 598 CLINICAL MICROBIOLOGY. (3) An introduction to the concepts of clinical microbiology through a survey of the microbial diseases of man using an organ system approach. Prereq: BIO 208 and 209, BIO 476G recommended, CHE 230 or 236, or consent of instructor. (Same as PAT 598.) MI 601 SPECIAL TOPICS IN MOLECULAR AND CELLULAR GENETICS. (1) Each semester five distinguished scientists visit the UK campus to deliver a series of three formal lectures each and participate in numerous informal contacts with graduate students. -

Level of Anti-FSH and Anti-LH Antibody in PCOS Women And
ry & Imm st u i n m o e p h a c t Hussein et al., Immunochem Immunopathol 2018, 4:1 o h o n Immunochemistry & l u o DOI: 10.4172/2469-9756.1000129 g m y m I ISSN: 2469-9756 Immunopathology ResearchResearch Article Article Open Access Level of Anti-FSH and Anti-LH Antibody in PCOS Women and Comparing it with Normal Control Group Shafaq Hussein*, Ihsan Al-Saimary and Maysoon Sherif Department of Microbiology, College of Medicine, University of Basrah, Iraq Abstract Background: Polycystic ovary syndrome is commonest endocrine disorder of female at reproductive age with estimation of its prevalence in general population was 20-33%. Objective: To estimate the levels of Anti-FSH Ab, Anti-LH Ab in polycystic women and comparing it with healthy control group. Patients and methods: This case controls study was carried out in Basrah. In this study blood sample from 29 women with PCOS and 29 healthy control women were collected from outpatient and private gynecological clinics and primary health care centers from different area of Basra from August 2016 to March 2018 for estimation of their serum levels of Anti-FSH Ab, Anti-LH Ab by using enzyme linked immunosorbent assay technique. Results: Serum levels of anti-FSH Ab, anti-LH Ab were elevated in PCOS women. Conclusion: Serum levels of anti-FSH Ab, anti-LH Ab is highly statistically significance in PCOS women than in healthy control group. Keywords: PCOS; Anti-FSH Ab; Anti-LH Ab; Serum levels comparing to normal control group. Introduction Materials and Methods Polycystic ovarian syndrome is a heterogeneous collection of Patients signs and symptoms that form a spectrum of a disorder with a mild A total number of 29 women with PCOS was involved in this case presentation in some women and a severe disturbance of reproductive, control study. -

Implications of Low-Grade Inflammation in SARS-Cov-2 Immunopathology
Review Article Implications of Low-grade Inflammation in SARS-CoV-2 Immunopathology Anamary Suárez-Reyes MD and Carlos A. Villegas-Valverde MD MS ABSTRACT senescent and obese patients and those with cardiovascular disease, INTRODUCTION Advanced age and chronic disease comorbidities cancer or chronic obstructive pulmonary disease. Low-grade chronic are indicators of poor prognosis in COVID-19 clinical progression. infl ammation is characteristic of all these conditions, refl ected in a pro- infl ammatory state, endothelial dysfunction, and changes to innate Fatal outcomes in patients with these characteristics are due to a immunity; mainly of the monocyte-macrophage system with changes dysfunctional immune response. Understanding COVID-19’s immu- in polarization, infl ammation, cytotoxicity and altered antigenic pre- nopathogenesis helps in designing strategies to prevent and mitigate sentation. In the case of SARS-CoV-2 infection, mechanisms involved complications during treatment. in acute infl ammation overlap with the patient’s pro-infl ammatory state, causing immune system dysfunction. SARS-CoV-2 infection OBJECTIVE Describe the main immunopathogenic alterations of amplifi es already-existing alterations, causing failures in the immune COVID-19 in patients of advanced age or with chronic non-commu- system’s control mechanisms. The resulting cytokine storm causes an nicable diseases. uncontrolled systemic infl ammatory response marked by high serum levels of infl ammatory biomarkers and a pro-infl ammatory cytokine DATA ACQUISITION We carried out a bibliographic search of pri- profi le with decompensation of underlying diseases. In asthma, chron- mary references in PubMed, Elsevier, Science Direct and SciELO. A ic eosinophilic infl ammation protects against infection by producing total of 270 articles met our initial search criteria. -

(P30) Test Because Spermatozoa Production
~. ANALYSIS OF SEMINAL FLUID IDENTIFICATION OF SEMEN-SPECIFIC PROTEIN p30 BY ABAcard@PSA (p30) Test Because spermatozoa production in males can be affected by aspermia, vasectomy or other conditions, forensic scientists have recognized the need for seminal fluid diagnostic tests which did not rely upon sperm cell presence. p30 is a 30,000 Dalton semen glycoprotein of prostatic origin that is also known as PSA or prostate specific antigen. The range of PSA is 200,000 to 5.5 million ng/ml of semen. The sensitivity of the ABAcard@PSA test is 4 ng/ml and therefore seminal fluid diluted up to 1 in a million should be detectable. The ABAcard@PSA test has been tested against numerous biological fluids of men and women. Since no cross reactivity has been reported to date, this supports the hypothesis that P30 is a male-specific protein. For this reason, the detection of the P30 antigen in a forensic stain is strong evidence that the stain is seminal in nature. The determination of the presence of semen may be made by using the ABAcard@ PSA (p30) test. In this test 200~1of sample is added to the sample well'S', and allowed to soak in. If PSA is present in the specimen, it will react with the mobile monoclonal ~ antihuman PSA antibody and a mobile antigen antibody complex is thus formed. This mobile antibody-antigen complex migrates through the absorbent device towards the test area 'T'. In the test area 'T', a polyclonal antihuman PSA antibody is immobilized. This immobilized antibody captures the above complex so that an antibody-antigen- antibody sandwich is formed. -

RF Calibrator (Ver.2), Disease Control, Atlanta, GA
KAMIYA BIOMEDICAL COMPANY further information, refer to "Decontamination of Laboratory Materials Required But Not Supplied Sink Drains to Remove Azide Salts," in the Manual Guide- K-ASSAY Safety Management No. CDC-22 issued by the Center for Calibrators: K-ASSAY RF Calibrator (Ver.2), Disease Control, Atlanta, GA. Cat. No. KAI-231C. RF (Ver.2) Purified water REAGENT PREPARATION For the Quantitative Determination of Human Rheumatoid Factor (RF) in Serum and Plasma Two Reagent Clinical Chemistry Analyzer: Reagents are ready to use and do not require reconstitution. Capable of accurate absorbance readings Cat. No. KAI-230 Capable of accurately dispensing the required volumes STORAGE AND HANDLING Capable of maintaining 37°C INTENDED USE calibrators with known concentrations of proteins and using the instrument’s data reduction capability or All reagents should be stored refrigerated (2-8°C). Return all Assay Procedure The K-ASSAY RF (Ver.2) assay is for the quantitative manually plotting the change in absorbance versus reagents to 2-8°C promptly after use. Unopened reagents determination of human IgG rheumatoid factor antibodies in concentration. Concentration of the controls and patient can be used until the expiration date indicated on the Note: Allow all reagents and specimens to warm to room patient serum or plasma (citric acid, EDTA, or lithium samples are interpolated from the calibration curve. package and bottle labels. temperature. Mix all reagents gently before using. heparin) based on immunoturbidimetric assay. The presence The K-ASSAY RF (Ver.2) assay should be run using the of IgG RF antibodies, in conjunction with clinical findings and REAGENT STABILITY An example of automated application: K-ASSAY RF Calibrator (Ver.2). -

٢Year Dr. Hiyam Adil Altaii Lec. ٣ IMMUNOPATHOLOGY
2Year Dr. Hiyam Adil Altaii Lec. 3 IMMUNOPATHOLOGY IMMUNOPATHOLOGY The infected tissue often serves as an innocent bystander and immunopathology results. This can occur in acute and chronic infections. Over stimulation of cytokine production and complement activation by endotoxins can cause tissue injury in the absence of an immune response. Continuously generated antigens released from persisting viable microbes will subsequently elicit humoral antibodies and cell mediated immunity resulting in chronic immunopathology. Certain poorly degradable antigens (e.g pneumococcal polysaccharide and group A streptococcal cell walls) can maintain immunopathology even in the absence of persistence of live agents. Other bacterial antigens cross-react with host tissue antigens causing the development of autoimmunity (e.g. the M protein of S. pyogenes cross-reacts with mammalian myosin). Thus immunopathology can persist even after the infection and microbial antigens are eliminated. The immune system in resistance to infection - examples 1. Extracellular parasites. Antibodies cause lysis of the organism and/or their opsonization by phagocytes at which point they are rapidly killed. 2. Intracellular parasites are primarily killed by cell mediated immunity. 3. Exotoxins can be neutralized by antitoxins. These can be elicited using toxoid vaccines (toxoids are antigenic but not toxic). This occurs, for example, in vaccination against diphtheria. 4. Certain organisms produce IgA proteases (including H. influenzae, S. pneumoniae, N. gonorrhoeae and N. -

Medical Microbiology and Immunology 1
Medical Microbiology and Immunology 1 MEDICAL MICROBIOLOGY AND Doctor of Philosophy (Ph.D.) Program The objective of the program is to prepare highly qualified students for IMMUNOLOGY a broad range of possible careers in research and teaching in medical microbiology and immunology and related health science fields. Study Program Director: Patrick C. Swanson, Ph.D. for the Ph.D. degree emphasizes independence in scientific pursuit, Program Office: Criss II, Room 529 with a particular emphasis on research. Course work and dissertation research are designed to bring the student to a high-level of competence Graduate Study in Medical Microbiology in microbiology and immunology with particular expertise in the area chosen for dissertation research. You will be expected to demonstrate and Immunology a high capacity for original and independent thought, and apply this Within the context of Creighton as a Jesuit, Catholic University, the creativity, educational background, and knowledge of the scientific Graduate School provides value-centered education for students to method to dissertation research. develop mastery of their chosen field of study. The Medical Microbiology and Immunology programs offer an environment ideal for fostering Master of Science (M.S.) Program critical judgment, scholarly initiative, and disciplined inquiry. The objectives of the program include preparation of the student for one or more of the following careers: Program Goals At the completion of this graduate program in Medical Microbiology & 1. teaching of medical microbiology and immunology at the Immunology, students will: undergraduate level, and 2. participation in supervised or team research in universities, industry 1. Demonstrate advanced knowledge in the fields of Medical or government.