Identification and Prevalence of Phascolarctid Gammaherpesvirus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
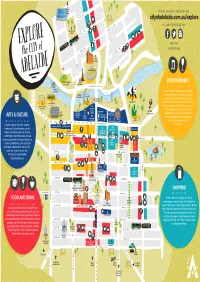
Cityofadelaide.Com.Au/Explore RYMILL NATIONAL NATIONAL
TO LE FEVRE TCE ADELAIDE AQUATIC FOR TIPS ON WHAT TO SEE AND DO VISIT CENTRE TYNTE ST cityofadelaide.com.au/explore FOLLOW CITYOFADELAIDE ON: WELLINGTON KINGSTON TCE SQUARE ARCHER ST O’CONNELL ST STANLEY ST #ADELAIDE #VISITADELAIDE WARD ST MELBOURNE ST ST PETER’S CATHEDRAL JEFFCOTT ST ADELAIDE OVAL NORTH ADELAIDE COLONEL ENTERTAINMENT GOLF COURSE LIGHT STATUE WAR MEMORIAL DRIVE TORRENS RIVER WEIR Wander through the yellow areas of the map TORRENS for a range of things to see and do for all ages, FOOTBRIDGE ROTUNDA including music, comedy, theatre, sport and POPEYE ELDER PARK ADELAIDE ZOO recreation. Check out a game at the Adelaide Oval, OLD ADELAIDE LAUNCH GAOL BONYTHON PARK take a cruise along the River Torrens, play a round PLAYSPACE ADELAIDE CASINO FESTIVAL at the North Adelaide Golf Course, visit the GOVERNMENT NATIONAL CENTRE ADELAIDE HOUSE MIGRATION WINE CENTRE Adelaide Zoo or catch a live band. There is SAHMRI CONVENTION NATIONAL MUSEUM RAILWAY ARTS & CULTURE CENTRE WAR MEMORIAL always something entertaining happening STATION BOTANIC PARLIAMENT AVE KINTORE ART GARDENS HOUSE LIBRARY MUSEUM GALLERY in the City, day and night and all NORTH TCE year round! A diverse range of museums, galleries, SAMSTAG MUSEUM LION ARTS CENTRE theatres and cultural landmarks can be MALLS BALLS AYERS HOUSE MALLS PIGS JAM FACTORY ST FROME found in the dark blue areas of the map. MERCURY CINEMA BANK ST GAWLER PL GAWLER ST PULTENEY HINDLEY ST MEDIA RESOURCE CENTRE A stroll through any of these areas will also RUNDLE MALL uncover quirky street art, famous statues and RUNDLE ST sculptures. -

Ecology of the Koala, Phascolarctos Cinereus
I give eonsent to this eopy of ny thesis, r,,rhen d.eposited. in the Universit.y Library, being avail-abl-e 1'or loan and. photocopying. Date . ?! ÛP,"+ .13:r.o.. S igned. CONTENTS SUM MA RY ACKNOWLEDGEMENTS lil INTRODUCTION I PA,RT I FIELD STUDIES INTRODUCTION O.l Kongoroo lslqnd B O.2 Floro ond Founo il 0.3 Philpott's Study l3 O.4 Methods t5 0.5 Results 25 I THE DISTRIBUTION AND ABUN DANCE OF KOALAS I. I The Distribution of Koalos 29 | .2 The Abundonce of Koo lqs 34 2 BREEDING, GROWTH AND DEVELOPA,\E¡.¡T 2.1 Breeding 39 2.2 Pouch Young 40 2.3 Growth, Ageing ond LongevitY 49 2.4 Sexucrl Moturity 54 I SUMMARY The distribution of koalas u'ithin Flinders Chase was fou-nd to be made up of areas centred on the occurrences of manna guilr , Euca.ly¡rtus viminalis. Some koalas br:owsed chiefly iri trees of other species but tlrere liÌere ferv animals, if any, that clid not feed on the foliage of E. r'iminalis rnore or less regularly. The composition of populations in sever¿rl sürcly areas changed from üirne to time but over aE long as three successir¡e years of observat:lorr the numhers remained ::emarkably constant. The koalas bred in the surnmer: arrd early auturnn, and a high proporüon of feinales successfully raised a single young to independence each year. Growth of the yourìg was :lapid over the first Lhree yearr!; it slowed. down thereafter and anirnals reached firll size in tlieir fourth and fiffh years. -

Platypus Collins, L.R
AUSTRALIAN MAMMALS BIOLOGY AND CAPTIVE MANAGEMENT Stephen Jackson © CSIRO 2003 All rights reserved. Except under the conditions described in the Australian Copyright Act 1968 and subsequent amendments, no part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, duplicating or otherwise, without the prior permission of the copyright owner. Contact CSIRO PUBLISHING for all permission requests. National Library of Australia Cataloguing-in-Publication entry Jackson, Stephen M. Australian mammals: Biology and captive management Bibliography. ISBN 0 643 06635 7. 1. Mammals – Australia. 2. Captive mammals. I. Title. 599.0994 Available from CSIRO PUBLISHING 150 Oxford Street (PO Box 1139) Collingwood VIC 3066 Australia Telephone: +61 3 9662 7666 Local call: 1300 788 000 (Australia only) Fax: +61 3 9662 7555 Email: [email protected] Web site: www.publish.csiro.au Cover photos courtesy Stephen Jackson, Esther Beaton and Nick Alexander Set in Minion and Optima Cover and text design by James Kelly Typeset by Desktop Concepts Pty Ltd Printed in Australia by Ligare REFERENCES reserved. Chapter 1 – Platypus Collins, L.R. (1973) Monotremes and Marsupials: A Reference for Zoological Institutions. Smithsonian Institution Press, rights Austin, M.A. (1997) A Practical Guide to the Successful Washington. All Handrearing of Tasmanian Marsupials. Regal Publications, Collins, G.H., Whittington, R.J. & Canfield, P.J. (1986) Melbourne. Theileria ornithorhynchi Mackerras, 1959 in the platypus, 2003. Beaven, M. (1997) Hand rearing of a juvenile platypus. Ornithorhynchus anatinus (Shaw). Journal of Wildlife Proceedings of the ASZK/ARAZPA Conference. 16–20 March. -

A Phylogeny and Timescale for Marsupial Evolution Based on Sequences for Five Nuclear Genes
J Mammal Evol DOI 10.1007/s10914-007-9062-6 ORIGINAL PAPER A Phylogeny and Timescale for Marsupial Evolution Based on Sequences for Five Nuclear Genes Robert W. Meredith & Michael Westerman & Judd A. Case & Mark S. Springer # Springer Science + Business Media, LLC 2007 Abstract Even though marsupials are taxonomically less diverse than placentals, they exhibit comparable morphological and ecological diversity. However, much of their fossil record is thought to be missing, particularly for the Australasian groups. The more than 330 living species of marsupials are grouped into three American (Didelphimorphia, Microbiotheria, and Paucituberculata) and four Australasian (Dasyuromorphia, Diprotodontia, Notoryctemorphia, and Peramelemorphia) orders. Interordinal relationships have been investigated using a wide range of methods that have often yielded contradictory results. Much of the controversy has focused on the placement of Dromiciops gliroides (Microbiotheria). Studies either support a sister-taxon relationship to a monophyletic Australasian clade or a nested position within the Australasian radiation. Familial relationships within the Diprotodontia have also proved difficult to resolve. Here, we examine higher-level marsupial relationships using a nuclear multigene molecular data set representing all living orders. Protein-coding portions of ApoB, BRCA1, IRBP, Rag1, and vWF were analyzed using maximum parsimony, maximum likelihood, and Bayesian methods. Two different Bayesian relaxed molecular clock methods were employed to construct a timescale for marsupial evolution and estimate the unrepresented basal branch length (UBBL). Maximum likelihood and Bayesian results suggest that the root of the marsupial tree is between Didelphimorphia and all other marsupials. All methods provide strong support for the monophyly of Australidelphia. Within Australidelphia, Dromiciops is the sister-taxon to a monophyletic Australasian clade. -

Event Information
BLANCO FOOD & EVENTS: RESTAURANT & CATERING AWARDS FOR EXCELLENCE National Event Caterer of the Year 2008 South Australian Event Caterer of the Year 2003, 2004, 2005, 2007, 2008, 2009 ENQUIRIES T. +61 8 8230 1313 PO Box 2669 South Australian Caterer of the Year 2003 [email protected] F. +61 8 8132 0813 Kent Town South Australian Hall of Fame 2006, 2010 www.blancofood.com.au South Australia 5071 South Australian Sanctuary Adelaide Zoo – Venue Caterer of the Year 2010, 2011 EVENT INFORMATION PLANE TREE DRIVE, ADELAIDE, SOUTH AUSTRALIA A BLANCO FOOD & EVENTS VENUE www.blancofood.com.au | [email protected] | +61 8 8230 1313 WELCOME Thank you for considering the Sanctuary Adelaide Zoo and the award winning Blanco Food & Events Team for your upcoming event. Opened in 2010, the Sanctuary Adelaide Zoo is a state of the art function facility located on the first floor of the Adelaide Zoo’s new $30 million re-development. This development has coincided with the arrival of Wang Wang and Funi – the only two giant pandas in the southern hemisphere. Sanctuary Adelaide Zoo provides the latest technology within flexible meeting, exhibition or banquet space over looking the magnificent parklands of Adelaide’s CBD fringe. Managed by Blanco Food & Events, our professional service and depth of experience is reflected in our multiple awards including “The Best Achievement in Catering” at the 2011 Australian Event Awards. Blanco’s partnership with Adelaide Zoo provides you with amazing animal encounters at your event which are unequalled. Giant pandas, tiger feeding, animal handling, can provide a memorable experience at your event. -

Reproductionreview
REPRODUCTIONREVIEW Wombat reproduction (Marsupialia; Vombatidae): an update and future directions for the development of artificial breeding technology Lindsay A Hogan1, Tina Janssen2 and Stephen D Johnston1,2 1Wildlife Biology Unit, Faculty of Science, School of Agricultural and Food Sciences, The University of Queensland, Gatton 4343, Queensland, Australia and 2Australian Animals Care and Education, Mt Larcom 4695, Queensland, Australia Correspondence should be addressed to L A Hogan; Email: [email protected] Abstract This review provides an update on what is currently known about wombat reproductive biology and reports on attempts made to manipulate and/or enhance wombat reproduction as part of the development of artificial reproductive technology (ART) in this taxon. Over the last decade, the logistical difficulties associated with monitoring a nocturnal and semi-fossorial species have largely been overcome, enabling new features of wombat physiology and behaviour to be elucidated. Despite this progress, captive propagation rates are still poor and there are areas of wombat reproductive biology that still require attention, e.g. further characterisation of the oestrous cycle and oestrus. Numerous advances in the use of ART have also been recently developed in the Vombatidae but despite this research, practical methods of manipulating wombat reproduction for the purposes of obtaining research material or for artificial breeding are not yet available. Improvement of the propagation, genetic diversity and management of wombat populations requires a thorough understanding of Vombatidae reproduction. While semen collection and cryopreservation in wombats is fairly straightforward there is currently an inability to detect, induce or synchronise oestrus/ovulation and this is an impeding progress in the development of artificial insemination in this taxon. -

Effects of Eucalypt Plant Monoterpenes on Koala
www.nature.com/scientificreports OPEN Efects of Eucalypt Plant Monoterpenes on Koala (Phascolarctos Cinereus) Cytokine Expression In Vitro Caroline Marschner*, Mark B. Krockenberger & Damien P. Higgins Protective immunity is crucial for survival of any species, though the koala as a specialist feeder adapted to an exclusive diet of eucalypts that contain plant secondary metabolites of varying toxicity and of immunomodulatory property. Being constantly exposed to such dietary chemicals it raises the question of their immune efects in a specialist eucalypt feeder. This study demonstrates that natural levels of circulating eucalypt plant secondary metabolites have dose dependent in vitro efects on cytokine expression of koala peripheral blood mononuclear cells, suggesting a potential trade-of of reduced function in multiple arms of the immune system associated with koala’s use of its specialized dietary niche. Widespread in the Australian landscape, eucalypts comprise an easily accessible food resource for some foli- vores, such as the koala (Phascolarctos cinereus), the ringtail possum (Pseudocheirus peregrinus) and greater glider (Petauroides volans) that are able to exploit this dietary niche. Te koala is able to utilise this resource exclusively for nutrition and shelter1 even though chemical defences render eucalypt leaves unpalatable, of low nutritional value, and even toxic. Tannin and lignin (up to 30% DM) bind valuable leaf proteins2,3 and cell wall carbohy- drates4 in the gastrointestinal tract of herbivores. Most leaf fats are either indigestible waxes2 or toxic terpenoids. Monoterpenes, an abundant group of terpenoids in eucalypts, are of small molecular weight and highly lipo- philic, hence are readily absorbable from the gut wall of eucalypt folivores5, and enter the circulation5–10. -

Download the World Routes 2019 Essential Guide to Adelaide
Your Essential Guide to Adelaide World Routes 2019 | 21 - 2 4 S eptember I A world of experiences at your fingertips in Adelaide. Adelaide is bursting with culture, flavours, events and entertainment. This vibrant and friendly city invites you to reward your curiosity and discover what makes Adelaide the perfect home for World Routes 2019. Adelaide Oval, Adelaide Your Essential Guide Welcome to Adelaide Welcome, from the hosts of World Routes 2019. Surrounded by lush parklands and speckled with an eclectic combination of historic buildings, trendy bars and state-of- the-art modern facilities, Adelaide is beckoning to be explored. We invite you to indulge in some of Australia’s most awarded restaurants in the heart of the city, immerse yourself in a thriving local arts scene, and unveil the oldest culture on earth through the world’s largest Aboriginal artefact collection. Adelaide, with its bustling Riverbank Precinct, and world-class venues such as the Adelaide Oval, Adelaide Showground and Adelaide Convention Centre, is an ideal setting for major events and conferences. The city can accommodate event-goers from around the world but remains compact enough to enable our visitors to roam freely, explore local attractions, and stay confident that their home base is never too far. Step outside of the city, soak up some sun and uncover a diverse array of experiences in our regions. Taste your way through world-famous wine regions only minutes from the city. Adelaide is a gateway to some of Australia’s best wine country and is recognised as a member of the prestigious Great Wine Capitals Global Network. -

Bearing up Well? Understanding the Past, Present and Future of Australia's Koalas
Gondwana Research 25 (2014) 1186–1201 Contents lists available at ScienceDirect Gondwana Research journal homepage: www.elsevier.com/locate/gr GR focus review Bearing up well? Understanding the past, present and future of Australia's koalas Karen H. Black a,⁎, Gilbert J. Price b, Michael Archer a, Suzanne J. Hand a a School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, New South Wales 2052, Australia b Department of Earth Sciences, University of Queensland, St Lucia, Queensland 4072, Australia article info abstract Article history: The modern Koala Phascolarctos cinereus is the last surviving member of a once diverse family Phascolarctidae Received 20 October 2013 (Marsupialia, Phascolarctomorphia). Nine genera and at least 16 species of koala are known. Late Oligocene sed- Received in revised form 17 December 2013 iments of central Australia record the oldest fossils and highest species diversity. Five species are known from the Accepted 22 December 2013 early to middle Miocene rainforest assemblages of the Riversleigh World Heritage Area, Queensland. With the Available online 30 December 2013 onset of dryer conditions after the middle Miocene climatic optimum (~16 Ma), rainforest habitats contracted Handling Editor: M. Santosh resulting in the apparent extinction of three koala lineages (Litokoala, Nimiokoala, Priscakoala). Phascolarctos first appears in the fossil record during the Pliocene and the modern species around 350 ka. Despite a dramatic Keywords: decline in taxonomic diversity to a -

Koalas and Climate Change
KOALAS AND CLIMATE CHANGE Hungry for CO2 cuts © Daniele Sartori Summary • Increasing frequency and intensity of droughts can force Koalas to descend from trees in search of water or • Koalas are iconic animals native to Australia. They new habitats. This makes them particularly vulnerable to are true habitat and food specialists, only ever inhabiting wild and domestic predators, as well as to road traffic, forests and woodlands where Eucalyptus trees are often resulting in death. present. • Koala populations are reported to be declining • Increasing atmospheric CO levels will reduce the 2 probably due to malnutrition, the sexually-transmitted nutritional quality of Eucalyptus leaves, causing nutrient disease chlamydia, and habitat destruction. shortages in the species that forage on them. As a result, Koalas may no longer be able to meet their nutritional • Koalas have very limited capability to adapt to rapid, demands, resulting in malnutrition and starvation. human-induced climate change, making them very vulnerable to its negative impacts. The IUCN Red List of Threatened Species ™ KOALAS AND CLIMATE CHANGE • Koalas are particularly vulnerable to the effects of elevated CO2 levels on plant nutritional quality, as they rely on them for food. The potential impacts of these changes on the world’s food chains are enormous. Australian icon, the Koala (Phascolarctos cinereus), is a tree-dwelling marsupial found in eastern and southern Australia. Marsupials are mammals whose young are born at a very undeveloped stage before completing their development in a pouch. The Koala is not a bear, though this name has persisted outside Australia since English- speaking settlers from the late 18th century likened it to a bear. -

Sarcoptes Scabiei: an Important Exotic Pathogen of Wombats
Under the Microscope Sarcoptes scabiei: an important exotic pathogen of wombats Sarcoptes scabiei is a parasitic astigmatid favourable for survival of the mite when mite, which causes scabies in people and Lee F Skerratt off the host. It is thought that the sarcoptic mange in mammals (Figure 1). School of Veterinary and duration of mite survival off the host is a Importantly, it is an emerging disease in Biomedical Sciences key component affecting transmission James Cook University, wildlife throughout the world 1. The mite Townsville 4811 between wombats because wombats are originates from a human ancestor and is Australia. generally antisocial and avoid contact thought to have spread to domestic and Tel: 617 4781 4838 with one another 9. Wombats rely on then free-living animals 2, 3. Based on the Fax: 617 4779 1526 burrows for diurnal shelter and recent emergence of sarcoptic mange in E-mail: [email protected] transmission may occur when wombats Australian wildlife and Aboriginal share burrows. Burrows enhance the communities, it is thought that Sarcoptes Epidemiology in wombat survival of mites when off the host by scabiei was probably introduced to populations providing a stable temperate Australia by the Europeans and their environment. animals 3,4. The mitochondrial genetic Sarcoptic mange generally occurs at low similarity of mites from Australian wildlife prevalence (0 - 15%) in common wombat Epidemics of sarcoptic mange occur and domestic animals supports this 3, 5. In populations throughout southeast sporadically within wombat populations 7,8 Australian wildlife, sarcoptic mange has Australia . Its low prevalence is and appear to be mainly associated with been reported in the common wombat attributed to high mortality and immunity introduction of S. -

High School Formal Packages
BLANCO FOOD & EVENTS: RESTAURANT & CATERING AWARDS FOR EXCELLENCE National Event Caterer of the Year 2008 South Australian Event Caterer of the Year 2003, 2004, 2005, 2007, 2008, 2009 ENQUIRIES T. +61 8 8230 1313 PO Box 2669 South Australian Caterer of the Year 2003 [email protected] F. +61 8 8132 0813 Kent Town South Australian Hall of Fame 2006, 2010 www.blancofood.com.au South Australia 5071 South Australian Sanctuary Adelaide Zoo – Venue Caterer of the Year 2010, 2011 South Australian Tourism Restaurant of the Year 2012 HIGH SCHOOL FORMAL PACKAGES PLANE TREE DRIVE, ADELAIDE, SOUTH AUSTRALIA A BLANCO FOOD & EVENTS VENUE www.blancofood.com.au | [email protected] | +61 8 8230 1313 WELCOME Opened in 2010, the Sanctuary Adelaide Zoo is a state of the art function facility located on the first floor of the Adelaide Zoo’s new $30 million re-development. Book your This development has coincided with the arrival of Wang Wang and Funi, the only two giant pandas in the southern School formal by hemisphere. March 2013 for Sanctuary Adelaide Zoo offers flexible banquet space for dining and cocktail style within the magnificent parklands of 2013 and receive Adelaide’s CBD fringe. Managed by Blanco Food Events, our depth of experience is gift prizes of: reflected in our constant return clientele and multiple awards. We are Restaurant and Catering, Venue Caterer of the Year 2010 and 2011 and Events Australia Best Achievement in Catering 2011. We are dedicated to making your school ● 3 Breakfast formal unique and special with the packages we offer.