Energy Density
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Energy Storage Technologies' Application
sustainability Review A Review of Energy Storage Technologies’ Application Potentials in Renewable Energy Sources Grid Integration Henok Ayele Behabtu 1,2,* , Maarten Messagie 1, Thierry Coosemans 1, Maitane Berecibar 1, Kinde Anlay Fante 2 , Abraham Alem Kebede 1,2 and Joeri Van Mierlo 1 1 Mobility, Logistics, and Automotive Technology Research Centre, Vrije Universiteit Brussels, Pleinlaan 2, 1050 Brussels, Belgium; [email protected] (M.M.); [email protected] (T.C.); [email protected] (M.B.); [email protected] (A.A.K.); [email protected] (J.V.M.) 2 Faculty of Electrical and Computer Engineering, Jimma Institute of Technology, Jimma University, Jimma P.O. Box 378, Ethiopia; [email protected] * Correspondence: [email protected]; Tel.: +32-485659951 or +251-926434658 Received: 12 November 2020; Accepted: 11 December 2020; Published: 15 December 2020 Abstract: Renewable energy sources (RESs) such as wind and solar are frequently hit by fluctuations due to, for example, insufficient wind or sunshine. Energy storage technologies (ESTs) mitigate the problem by storing excess energy generated and then making it accessible on demand. While there are various EST studies, the literature remains isolated and dated. The comparison of the characteristics of ESTs and their potential applications is also short. This paper fills this gap. Using selected criteria, it identifies key ESTs and provides an updated review of the literature on ESTs and their application potential to the renewable energy sector. The critical review shows a high potential application for Li-ion batteries and most fit to mitigate the fluctuation of RESs in utility grid integration sector. -

Fuel Properties Comparison
Alternative Fuels Data Center Fuel Properties Comparison Compressed Liquefied Low Sulfur Gasoline/E10 Biodiesel Propane (LPG) Natural Gas Natural Gas Ethanol/E100 Methanol Hydrogen Electricity Diesel (CNG) (LNG) Chemical C4 to C12 and C8 to C25 Methyl esters of C3H8 (majority) CH4 (majority), CH4 same as CNG CH3CH2OH CH3OH H2 N/A Structure [1] Ethanol ≤ to C12 to C22 fatty acids and C4H10 C2H6 and inert with inert gasses 10% (minority) gases <0.5% (a) Fuel Material Crude Oil Crude Oil Fats and oils from A by-product of Underground Underground Corn, grains, or Natural gas, coal, Natural gas, Natural gas, coal, (feedstocks) sources such as petroleum reserves and reserves and agricultural waste or woody biomass methanol, and nuclear, wind, soybeans, waste refining or renewable renewable (cellulose) electrolysis of hydro, solar, and cooking oil, animal natural gas biogas biogas water small percentages fats, and rapeseed processing of geothermal and biomass Gasoline or 1 gal = 1.00 1 gal = 1.12 B100 1 gal = 0.74 GGE 1 lb. = 0.18 GGE 1 lb. = 0.19 GGE 1 gal = 0.67 GGE 1 gal = 0.50 GGE 1 lb. = 0.45 1 kWh = 0.030 Diesel Gallon GGE GGE 1 gal = 1.05 GGE 1 gal = 0.66 DGE 1 lb. = 0.16 DGE 1 lb. = 0.17 DGE 1 gal = 0.59 DGE 1 gal = 0.45 DGE GGE GGE Equivalent 1 gal = 0.88 1 gal = 1.00 1 gal = 0.93 DGE 1 lb. = 0.40 1 kWh = 0.027 (GGE or DGE) DGE DGE B20 DGE DGE 1 gal = 1.11 GGE 1 kg = 1 GGE 1 gal = 0.99 DGE 1 kg = 0.9 DGE Energy 1 gallon of 1 gallon of 1 gallon of B100 1 gallon of 5.66 lb., or 5.37 lb. -

Re-Examining the Role of Nuclear Fusion in a Renewables-Based Energy Mix
Re-examining the Role of Nuclear Fusion in a Renewables-Based Energy Mix T. E. G. Nicholasa,∗, T. P. Davisb, F. Federicia, J. E. Lelandc, B. S. Patela, C. Vincentd, S. H. Warda a York Plasma Institute, Department of Physics, University of York, Heslington, York YO10 5DD, UK b Department of Materials, University of Oxford, Parks Road, Oxford, OX1 3PH c Department of Electrical Engineering and Electronics, University of Liverpool, Liverpool, L69 3GJ, UK d Centre for Advanced Instrumentation, Department of Physics, Durham University, Durham DH1 3LS, UK Abstract Fusion energy is often regarded as a long-term solution to the world's energy needs. However, even after solving the critical research challenges, engineer- ing and materials science will still impose significant constraints on the char- acteristics of a fusion power plant. Meanwhile, the global energy grid must transition to low-carbon sources by 2050 to prevent the worst effects of climate change. We review three factors affecting fusion's future trajectory: (1) the sig- nificant drop in the price of renewable energy, (2) the intermittency of renewable sources and implications for future energy grids, and (3) the recent proposition of intermediate-level nuclear waste as a product of fusion. Within the scenario assumed by our premises, we find that while there remains a clear motivation to develop fusion power plants, this motivation is likely weakened by the time they become available. We also conclude that most current fusion reactor designs do not take these factors into account and, to increase market penetration, fu- sion research should consider relaxed nuclear waste design criteria, raw material availability constraints and load-following designs with pulsed operation. -

Energy and the Hydrogen Economy
Energy and the Hydrogen Economy Ulf Bossel Fuel Cell Consultant Morgenacherstrasse 2F CH-5452 Oberrohrdorf / Switzerland +41-56-496-7292 and Baldur Eliasson ABB Switzerland Ltd. Corporate Research CH-5405 Baden-Dättwil / Switzerland Abstract Between production and use any commercial product is subject to the following processes: packaging, transportation, storage and transfer. The same is true for hydrogen in a “Hydrogen Economy”. Hydrogen has to be packaged by compression or liquefaction, it has to be transported by surface vehicles or pipelines, it has to be stored and transferred. Generated by electrolysis or chemistry, the fuel gas has to go through theses market procedures before it can be used by the customer, even if it is produced locally at filling stations. As there are no environmental or energetic advantages in producing hydrogen from natural gas or other hydrocarbons, we do not consider this option, although hydrogen can be chemically synthesized at relative low cost. In the past, hydrogen production and hydrogen use have been addressed by many, assuming that hydrogen gas is just another gaseous energy carrier and that it can be handled much like natural gas in today’s energy economy. With this study we present an analysis of the energy required to operate a pure hydrogen economy. High-grade electricity from renewable or nuclear sources is needed not only to generate hydrogen, but also for all other essential steps of a hydrogen economy. But because of the molecular structure of hydrogen, a hydrogen infrastructure is much more energy-intensive than a natural gas economy. In this study, the energy consumed by each stage is related to the energy content (higher heating value HHV) of the delivered hydrogen itself. -

Ces-Collins-Pp-Ev-Conundrum
Gabriel Collins, J.D. Baker Botts Fellow for Energy & Environmental Regulatory Affairs Baker Institute for Public Policy, Rice University EVs = Power-to-WeigHt Ratio of an Aircraft, Onboard Fuel of a Subcompact Car • Electric veHicles’ instant torque and higH horsepower make the higher-end editions true performance monsters. Their power-to-weigHt ratios are often on par witH turboprop aircraft and many helicopters. • The fly in the ointment comes from the fact that batteries remain heavy and relative to hydrocarbons and simply cannot store energy nearly as efficiently per unit of mass. • The power-to-weigHt/onboard energy density relationsHip of the Rivian R1T electric truck and A-29 Big, long-range Super Tucano attack aircraft is sucH that platforms “Rivianizing” the Tucano would mean making its onboard jet fuel stores retain their current energy Super Tucano (17) and Rivian R1T (14) content, but weigH more per liter than lead metal. Needless to say, the plane’s fligHt endurance and carrying capacity would fall dramatically. • This estimate assumes the A-29’s PT-6 turboprop has a thermal efficiency of about 35%--less than half the R1T’s likely capacity to convert fuel into actual propulsive energy. Please cite as: Gabriel Collins, “The EV Conundrum: High Power Density and Low Energy Density,” Baker Institute ResearcH Source: Beechcraft, Car & Driver, EIA, EV Database, GE, Global Security, Man, Nikola, Peterbuilt, Rivian, SNC, Trucks.com, USAF, US Navy Presentation, 8 January 2020, Houston, TX Gabriel Collins, J.D. Baker Botts Fellow for Energy & Environmental Regulatory Affairs Baker Institute for Public Policy, Rice University Electric Vehicles’ Onboard Energy Still Significantly Trails ICE Vehicles On an Efficiency-Adjusted Basis Efficiency-Adjusted Comparison • To make the energy density comparison a bit more fair, we adjust the onboard fuel based on the fact that EVs convert nearly 80% of their battery energy into tractive power at the wheels, while IC vehicles feature efficiencies closer to 20%. -

Sodium‐Ion Batteries Paving the Way for Grid Energy Storage
ESSAY 10th Anniversary Article www.advenergymat.de Sodium-Ion Batteries Paving the Way for Grid Energy Storage Hayley S. Hirsh, Yixuan Li, Darren H. S. Tan, Minghao Zhang, Enyue Zhao, and Y. Shirley Meng* Dedicated to the pioneering scientists whose work have made sodium-ion batteries possible bridge the disconnect between renewables The recent proliferation of renewable energy generation offers mankind hope, generation and distribution for consump- with regard to combatting global climate change. However, reaping the full tion. While stationary storage such as benefits of these renewable energy sources requires the ability to store and pumped hydroelectric and compressed air exist, their lack of flexible form factors and distribute any renewable energy generated in a cost-effective, safe, and sus- lower energy efficiencies limit their scal- tainable manner. As such, sodium-ion batteries (NIBs) have been touted as able adoption for urban communities.[2] an attractive storage technology due to their elemental abundance, promising Thus, batteries are believed to be more electrochemical performance and environmentally benign nature. Moreover, practical for large-scale energy storage new developments in sodium battery materials have enabled the adoption of capable of deployment in homes, cities, high-voltage and high-capacity cathodes free of rare earth elements such as Li, and locations far from the grid where the traditional electrical infrastructure does Co, Ni, offering pathways for low-cost NIBs that match their lithium coun- not reach. terparts in energy density while serving the needs for large-scale grid energy Today’s battery technologies are domi- storage. In this essay, a range of battery chemistries are discussed alongside nated by lithium ion batteries (LIBs) and their respective battery properties while keeping metrics for grid storage in lead acid batteries. -

1 Volumetric Energy Density of Compressed Air and Compressed
Volumetric Energy Density of Compressed Air and Compressed Methane. Volumetric energy density is a combination of the potential for mechanical work, w, done by the change in pressure (P), and volume (V), and the chemical heat, q, released from burning the gas. For example, compressed air at 2,900 psi (~197 atm) has an energy density of 0.1 MJ/L calculated from PV and compressed methane (at 2,900 psi) has an energy density of 8.0 MJ/L calculated from the combination of PV and heat of combustion (Eq. 1). Estimate of Pressure Immediately After Ignition. Using the unpressurized channel volume of 1.0 mL, and our theoretically estimated temperature immediately after ignition of 2,800 oC, we calculated the maximum pressure, using the ideal gas law, to be ~1 MPa (~140 psi). Though this value is an overestimate (it does not take into account the channel volume expansion and the gas cooling, a complex calculation that is beyond the scope of this paper), we use it to illustrate the quick impulse of high pressure to use a passive valving system to control the flow of gas out of the channel after combustion and cooling. Fabrication of the Robot. The elastomer we used for the actuation layer was a stiff silicone rubber (Dragon Skin 10, DS-10; Smooth-on, Inc.). This elastomer has a greater Young’s modulus than our previous choice for pneumatic actuation (Ecoflex 00-30; Smooth-on, Inc.); pneu-nets composed of DS-10 could withstand the large forces generated within the channels during the explosion better than Ecoflex; in addition, DS-10 has a high resilience, which allowed the pneu-nets to release stored elastic energy rapidly for propulsion (Fig. -

Grid Energy Storage
Grid Energy Storage U.S. Department of Energy December 2013 Acknowledgements We would like to acknowledge the members of the core team dedicated to developing this report on grid energy storage: Imre Gyuk (OE), Mark Johnson (ARPA-E), John Vetrano (Office of Science), Kevin Lynn (EERE), William Parks (OE), Rachna Handa (OE), Landis Kannberg (PNNL), Sean Hearne & Karen Waldrip (SNL), Ralph Braccio (Booz Allen Hamilton). Table of Contents Acknowledgements ....................................................................................................................................... 1 Executive Summary ....................................................................................................................................... 4 1.0 Introduction .......................................................................................................................................... 7 2.0 State of Energy Storage in US and Abroad .......................................................................................... 11 3.0 Grid Scale Energy Storage Applications .............................................................................................. 20 4.0 Summary of Key Barriers ..................................................................................................................... 30 5.0Energy Storage Strategic Goals .......................................................................................................... 32 6.0 Implementation of its Goals ............................................................................................................... -
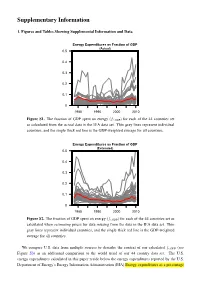
Comparing World Economic and Net Energy Metrics
Supplementary Information 1. Figures and Tables Showing Supplemental Information and Data Energy Expenditures as Fraction of GDP (Actual) 0.5 0.4 0.3 0.2 0.1 0 1980 1990 2000 2010 Figure S1. The fraction of GDP spent on energy (fe;GDP) for each of the 44 countries set as calculated from the actual data in the IEA data set. Thin gray lines represent individual countries, and the single thick red line is the GDP-weighted average for all countries. Energy Expenditures as Fraction of GDP (Estimated) 0.5 0.4 0.3 0.2 0.1 0 1980 1990 2000 2010 Figure S2. The fraction of GDP spent on energy (fe;GDP) for each of the 44 countries set as calculated when estimating prices for data missing from the data in the IEA data set. Thin gray lines represent individual countries, and the single thick red line is the GDP-weighted average for all countries. We compare U.S. data from multiple sources to describe the context of our calculated fe;GDP (see Figure S3) as an additional comparison to the world trend of our 44 country data set. The U.S. energy expenditures calculated in this paper reside below the energy expenditures reported by the U.S. Department of Energy’s Energy Information Administration (EIA) Energy expenditures as a percentage S2 of GDP from Table 1.5 and 3.5 of the Monthly or Annual Energy Review. From Tables 3.5 of Annual Energy Review: “Expenditures for primary energy and retail electricity by the four end-use sectors (residential, commercial, industrial, and transportation); excludes expenditures for energy by the electric power sector.” and approximately equal to slightly above personal consumption expenditures for energy goods and services as reported by the U.S. -

The Nuclear Option
proponents acknowledge. Humankind, The Nuclear Smil recounts, has experienced three major energy transitions: from wood Option and dung to coal, then to oil, and then to natural gas. Each took an extremely long time, and none is yet complete. Renewables Can’t Save the Nearly two billion people still rely on Planet—but Uranium Can wood and dung for heating and cooking. “Although the sequence of the three Michael Shellenberger substitutions does not mean that the fourth transition, now in its earliest stage (with fossil fuels being replaced by new conversions of renewable energy Energy and Civilization: A History flows), will proceed at a similar pace,” BY VACLAV SMIL. MIT Press, 2017, Smil writes, “the odds are highly in 552 pp. favor of another protracted process.” In 2015, even after decades of heavy round the world, the transition government subsidies, solar and wind from fossil fuels to renewable power provided only 1.8 percent of global A sources of energy appears to energy. To complete the transition, finally be under way. Renewables were renewables would need to both supply first promoted in the 1960s and 1970s as the world’s electricity and replace fossil a way for people to get closer to nature and fuels used in transportation and in the for countries to achieve energy indepen- manu facture of common materials, such dence. Only recently have people come as cement, plastics, and ammonia. Smil to see adopting them as crucial to pre- expresses his exasperation at “techno- venting global warming. And only in the optimists [who] see a future of unlimited last ten years has the proliferation of energy, whether from superefficient solar and wind farms persuaded much [photovoltaic] cells or from nuclear fusion.” of the public that such a transition is Such a vision, he says, is “nothing but possible. -

Electricity Storage and Renewables: Costs and Markets to 2030
ELECTRICITY STORAGE AND RENEWABLES: COSTS AND MARKETS TO 2030 October 2017 www.irena.org ELECTRICITY STORAGE AND RENEWABLES: COSTS AND MARKETS TO 2030 © IRENA 2017 Unless otherwise stated, material in this publication may be freely used, shared, copied, reproduced, printed and/or stored, provided that appropriate acknowledgement is given of IRENA as the source and copyright holder. Material in this publication that is attributed to third parties may be subject to separate terms of use and restrictions, and appropriate permissions from these third parties may need to be secured before any use of such material. ISBN 978-92-9260-038-9 Citation: IRENA (2017), Electricity Storage and Renewables: Costs and Markets to 2030, International Renewable Energy Agency, Abu Dhabi. About IRENA The International Renewable Energy Agency (IRENA) is an intergovernmental organisation that supports countries in their transition to a sustainable energy future, and it serves as the principal platform for international co-operation, a centre of excellence, and a repository of policy, technology, resource and financial knowledge on renewable energy. IRENA promotes the widespread adoption and sustainable use of all forms of renewable energy, including bioenergy, geothermal, hydropower, ocean, solar and wind energy, in the pursuit of sustainable development, energy access, energy security and low-carbon economic growth and prosperity. www.irena.org Acknowledgements IRENA is grateful for the the reviews and comments of numerous experts, including Mark Higgins (Strategen Consulting), Akari Nagoshi (NEDO), Jens Noack (Fraunhofer Institute for Chemical Technology ICT), Kai-Philipp Kairies (Institute for Power Electronics and Electrical Drives, RWTH Aachen University), Samuel Portebos (Clean Horizon), Keith Pullen (City, University of London), Oliver Schmidt (Imperial College London, Grantham Institute - Climate Change and the Environment), Sayaka Shishido (METI) and Maria Skyllas-Kazacos (University of New South Wales). -

Introduction to Fusion Energy
Introduction to Fusion Energy Jerry Hughes IAP @ PSFC January 8, 2013 Acknowledgments: Catherine Fiore, Jeff Freidberg, Martin Greenwald, Zach Hartwig, Alberto Loarte, Bob Mumgaard, Geoff Olynyk Presenter’s e-mail: [email protected] Questions to answer • What is fusion? • Why do we need it? • How do we get it on earth? • Where do we stand? • Where are we headed? What is fusion, anyway? What is fusion, anyway? What is fusion, anyway? What is fusion, anyway? Fusion is a form of nuclear energy E mc2 • A huge amount of energy is released when isotopes lighter than iron combine to form heavier nuclei, with less final mass • It is an ubiquitous energy source in the universe • It is not (yet) a practical energy source on earth Fusion is a form of nuclear energy E mc2 • A huge amount of energy is released when isotopes lighter than iron combine to form heavier nuclei, with less final mass • It is an ubiquitous energy source in the universe • It is not (yet) a practical energy source on earth Terrestrial energy sources have their origin in the nuclear fusion reactions of stars Supernova produces radioactive elements Solar heating of the Earth drives atmospheric circulation, water cycle Sun illuminates Earth Terrestrial energy sources have their origin in the nuclear fusion reactions of stars Geothermal Decay of radioactive particles generates heat in Earth’s interior Nuclear fission Supernova produces radioactive elements Splitting radioactive particles generates heat Solar heating of the Earth drives atmospheric circulation, water cycle