New Drug Arrivals Q 4 – Monograph Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients
antibodies Review Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients Andrew T. Lucas 1,2,3,*, Ryan Robinson 3, Allison N. Schorzman 2, Joseph A. Piscitelli 1, Juan F. Razo 1 and William C. Zamboni 1,2,3 1 University of North Carolina (UNC), Eshelman School of Pharmacy, Chapel Hill, NC 27599, USA; [email protected] (J.A.P.); [email protected] (J.F.R.); [email protected] (W.C.Z.) 2 Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] 3 Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-919-966-5242; Fax: +1-919-966-5863 Received: 30 November 2018; Accepted: 22 December 2018; Published: 1 January 2019 Abstract: The rapid advancement in the development of therapeutic proteins, including monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), has created a novel mechanism to selectively deliver highly potent cytotoxic agents in the treatment of cancer. These agents provide numerous benefits compared to traditional small molecule drugs, though their clinical use still requires optimization. The pharmacology of mAbs/ADCs is complex and because ADCs are comprised of multiple components, individual agent characteristics and patient variables can affect their disposition. To further improve the clinical use and rational development of these agents, it is imperative to comprehend the complex mechanisms employed by antibody-based agents in traversing numerous biological barriers and how agent/patient factors affect tumor delivery, toxicities, efficacy, and ultimately, biodistribution. -

Chemokine Receptors in Allergic Diseases Laure Castan, A
Chemokine receptors in allergic diseases Laure Castan, A. Magnan, Grégory Bouchaud To cite this version: Laure Castan, A. Magnan, Grégory Bouchaud. Chemokine receptors in allergic diseases. Allergy, Wiley, 2017, 72 (5), pp.682-690. 10.1111/all.13089. hal-01602523 HAL Id: hal-01602523 https://hal.archives-ouvertes.fr/hal-01602523 Submitted on 11 Jul 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - ShareAlike| 4.0 International License Allergy REVIEW ARTICLE Chemokine receptors in allergic diseases L. Castan1,2,3,4, A. Magnan2,3,5 & G. Bouchaud1 1INRA, UR1268 BIA; 2INSERM, UMR1087, lnstitut du thorax; 3CNRS, UMR6291; 4Universite de Nantes; 5CHU de Nantes, Service de Pneumologie, Institut du thorax, Nantes, France To cite this article: Castan L, Magnan A, Bouchaud G. Chemokine receptors in allergic diseases. Allergy 2017; 72: 682–690. Keywords Abstract asthma; atopic dermatitis; chemokine; Under homeostatic conditions, as well as in various diseases, leukocyte migration chemokine receptor; food allergy. is a crucial issue for the immune system that is mainly organized through the acti- Correspondence vation of bone marrow-derived cells in various tissues. Immune cell trafficking is Gregory Bouchaud, INRA, UR1268 BIA, rue orchestrated by a family of small proteins called chemokines. -

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

Biologic Armamentarium in Psoriasis
Vol 9, Issue 1, 2016 ISSN - 0974-2441 Review Article BIOLOGIC ARMAMENTARIUM IN PSORIASIS GANESH PAI1*, NITHIN SASHIDHARAN2 1Medical Director, Derma-Care ‘The Trade Centre’, Mangalore - 575 003, Karnataka, India. 2Consultant Clinical Pharmacologist, Derma-Care ‘The Trade Centre’, Mangalore - 575 003, Karnataka, India. Email: [email protected] Received: 14 July 2015, Revised and Accepted: 24 August 2015 ABSTRACT Psoriasis is an autoimmune disease and further classed as a chronic inflammatory skin condition serving as a global burden. A moderate to severe psoriasis can be treated with conventional therapies. Less efficacy, poor patient compliance, and toxicity issues were the major problems associated with conventional therapies. The introduction of biologic therapy has a great impression on psoriatic treatment duration and enhanced quality of life in psoriasis patients. The new biologic therapies are tailor-made medications with the goal of more specific and effective treatment; less toxicity. The biologic therapy is aimed to target antigen presentation and co-stimulation, T-cell activation, and leukocyte adhesion; and pro-inflammatory cascade. They act as effective and safer substitute to traditional therapy. Secukinumab, certolizumab, itolizumab, golimumab, ustekinumab, adalimumab, infliximab etanercept, alefacept, etc. are the approved biologic with the global market. This review briefs about psoriasis pathogenesis, traditional treatments, and biologic therapies potential. Keywords: Psoriasis, Biologic, Non-biologic treatment. INTRODUCTION migration, potentiation of Th1 type of response, angiogenesis, and epidermal hyperplasia [7]. Psoriasis is an autoimmune disease and further classed as a chronic inflammatory skin condition with prevalence ranging 1-3% in the TNF- is plays vital role in the pathogenesis of psoriasis. It acts by world [1]. -

Safety and Efficacy Profile of Mogamulizumab (Poteligeo)
Zhang et al. BMC Cancer (2021) 21:618 https://doi.org/10.1186/s12885-021-08363-w RESEARCH ARTICLE Open Access Safety and efficacy profile of mogamulizumab (Poteligeo) in the treatment of cancers: an update evidence from 14 studies Ting Zhang1,2†, Jing Sun3†, Jinying Li4, Yunuo Zhao1,2, Tao Zhang1,2, Ruoning Yang1,2 and Xuelei Ma1* Abstract Background: CC chemokine receptor 4 (CCR4), the receptor for CCL22 and CCL17, is expressed on the surface of effector Tregs that have the highest suppressive effects on antitumor immune response. CCR4 is also widely expressed on the surface of tumor cells from patients with adult T-cell leukemia/lymphoma (ATL), peripheral T-cell lymphoma (PTCL) and cutaneous T-cell lymphoma (CTCL). Mogamulizumab is a humanized, IgG1 kappa monoclonal antibody that is directed against CCR4. By reducing the number of CCR4-positive Tregs and tumor cells, the mogamulizumab can reduce tumor burden and boost antitumor immunity to achieve antitumor effects. Methods: We examined the PubMed and ClinicalTrials.gov until 1 February 2020. Considering variability in different studies, we selected the adverse events (AEs), overall survival (OS), progression-free survival (PFS), objective responses rate (ORR) and Hazard Ratio (HR) for PFS to evaluate the safety and efficacy profile of mogamulizumab. Results: When patients were treated with mogamulizumab monotherapy, the most common all-grade AEs were lymphopenia, infusion reaction, fever, rash and chills while the most common grade ≥ 3 AEs were lymphopenia, neutropenia and rash. When patients were treated with combined therapy of mogamulizumab and other drugs, the most common all-grade AEs were neutropenia, anaemia, lymphopenia and gastrointestinal disorder, while the most common grade ≥ 3 AEs was lymphopenia. -

Keeping up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A
Keeping Up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A. Shlom, PharmD, BCPS Senior Vice President & Director Clinical Pharmacy Program | Acurity, Inc. Privileged and Confidential April 10, 2019 Privileged and Confidential Program Objectives By the end of the presentation, the pharmacist or pharmacy technician participant will be able to: ▪ Identify orphan drugs and first-in-class medications approved by the FDA in 2018. ▪ List five new drugs and their indications. ▪ Identify the place in therapy for three novel monoclonal antibodies. ▪ Discuss at least two new medications that address public health concerns. Dr. Shlom does not have any conflicts of interest in regard to this presentation. Both trade names and generic names will be discussed throughout the presentation Privileged and Confidential 2018 NDA Approvals (NMEs/BLAs) ▪ Lutathera (lutetium Lu 177 dotatate) ▪ Braftovi (encorafenib) ▪ Vizimpro (dacomitinib) ▪ Biktarvy (bictegravir, emtricitabine, ▪ TPOXX (tecovirimat) ▪ Libtayo (cemiplimab-rwic) tenofovir, ▪ Tibsovo (ivosidenib) ▪ Seysara (sarecycline) alafenamide) ▪ Krintafel (tafenoquine) ▪ Nuzyra (omadacycline) ▪ Symdeko (tezacaftor, ivacaftor) ▪ Orilissa (elagolix sodium) ▪ Revcovi (elapegademase-lvir) ▪ Erleada (apalutamide) ▪ Omegaven (fish oil triglycerides) ▪ Tegsedi (inotersen) ▪ Trogarzo (ibalizumab-uiyk) ▪ Mulpleta (lusutrombopag) ▪ Talzenna (talazoparib) ▪ Ilumya (tildrakizumab-asmn) ▪ Poteligeo (mogamulizumab-kpkc) ▪ Xofluza (baloxavir marboxil) ▪ Tavalisse (fostamatinib disodium) ▪ Onpattro (patisiran) -

On the Horizon: Immuno-Oncology (I-O) Combinations
Immuno-Oncology (I-O) Combinations • Jeffrey A. Sosman, MD • Robert H. Lurie Comprehensive Cancer Center of Northwestern University The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 Stimulatory and Inhibitory Factors in the Cancer-Immunity Cycle Each step of the Cancer-Immunity Cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors shown in green promote immunity, ... Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Block other co-inhibitory: LAG3, TIM3, KIR, VISTA • Activate co-stimulatory: 4-1BB, OX-40, GITR, CD27, ICOS • Block inhibitory molecules- IDOi, TGFbi, CSF1Ri, anti-IL-6 or anti- IL-10 • Effect trafficking- anti-VEGF, CCL5, CXCR4i • Vaccines- TVEC- oncolytic virus, Neoantigen, other cellular • Adoptive Cellular therapy- TIL, CAR-T cells, TCR T-cells Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Signal Inhibition, BRAF directed (BRAFi+MEKi), MEKi, PI3K inhibition (PTEN effects) • Cytokines- IL-2, IFN a,b,g,, Directed cytokines (FAP-IL-2v or CEA-IL-2v) • Epigenetic modulation- gene expression and EVR expression • Microbiome modification- fecal transplants • Chemotherapy other cytotoxics • Localized Irradiation SBRT, SRS T cells in Tumors Express Multiple Immunoinhibitory Receptors -
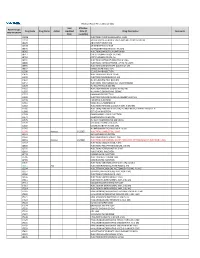
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody
Published OnlineFirst August 27, 2019; DOI: 10.1158/1078-0432.CCR-19-1090 Clinical Trials: Immunotherapy Clinical Cancer Research A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors Toshihiko Doi1, Kei Muro2, Hiroshi Ishii3, Terufumi Kato4, Takahiro Tsushima5, Mitsuhiro Takenoyama6, Satoshi Oizumi7, Kazuto Gemmoto8, Hideaki Suna8, Kouki Enokitani9, Tetsuyoshi Kawakami9, Hiroyoshi Nishikawa10,11, and Noboru Yamamoto12 Abstract Purpose: Regulatory T cells (Tregs) expressing CC chemo- part and 90 in the expansion part. No dose-limiting kine receptor 4 (CCR4) can suppress antitumor immune toxicities were observed in the dose-escalation part. Grade responses and are associated with poor prognoses in several 3/4 treatment-related adverse events (TRAEs) occurred in cancers. We assessed the safety and efficacy of combined 29% of patients in the expansion part (no grade 5 TRAEs). mogamulizumab (anti-CCR4 antibody) and nivolumab The most frequent TRAEs were rash (39%), rash maculopap- [anti-programmed death-1 (PD-1) antibody] in immunother- ular (20%), diarrhea (13%), stomatitis (12%), and pruritus apy-na€ve patients with advanced/metastatic solid tumors. (11%). There were four (27%) confirmed tumor responses Patients and Methods: This study (NCT02476123) com- among 15 patients with hepatocellular carcinoma, and prised dose-escalation (3þ3 design) and expansion parts. one confirmed and two unconfirmed responses among 15 Patients received nivolumab (3.0 mg/kg) every 2 weeks, with patients with pancreatic adenocarcinoma. During treatment, þ À mogamulizumab (0.3 or 1.0 mg/kg in dose escalation, populations of effector Tregs (CD4 CD45RA FoxP3high) þ 1.0 mg/kg in expansion) once weekly for 4 weeks, then every decreased and CD8 T cells in tumor-infiltrating lymphocytes 2 weeks, until progression or unacceptable toxicity. -

Immunfarmakológia Immunfarmakológia
Gergely: Immunfarmakológia Immunfarmakológia Prof Gergely Péter Az immunpatológiai betegségek döntő többsége gyulladásos, és ennek következtében általában szövetpusztulással járó betegség, melyben – jelenleg – a terápia alapvetően a gyulladás csökkentésére és/vagy megszűntetésére irányul. Vannak kizárólag gyulladásgátló gyógyszereink és vannak olyanok, amelyek az immunreakció(k) bénításával (=immunszuppresszió révén) vagy emellett vezetnek a gyulladás mérsékléséhez. Mind szerkezetileg, mind hatástanilag igen sokféle csoportba oszthatók, az alábbi felosztás elsősorban didaktikus célokat szolgál. 1. Nem-szteroid gyulladásgátlók (‘nonsteroidal antiinflammatory drugs’ NSAID) 2. Kortikoszteroidok 3. Allergia-elleni szerek (antiallergikumok) 4. Sejtoszlás-gátlók (citosztatikumok) 5. Nem citosztatikus hatású immunszuppresszív szerek 6. Egyéb gyulladásgátlók és immunmoduláns szerek 7. Biológiai terápia 1. Nem-szteroid gyulladásgátlók (NSAID) Ezeket a vegyületeket, melyek őse a szalicilsav (jelenleg, mint acetilszalicilsav ‘aszpirin’ használatos), igen kiterjedten alkalmazzák a reumatológiában, az onkológiában és az orvostudomány szinte minden ágában, ahol fájdalom- és lázcsillapításra van szükség. Egyes felmérések szerint a betegek egy ötöde szed valamilyen NSAID készítményt. Szerkezetük alapján a készítményeket több csoportba sorolhatjuk: szalicilátok (pl. acetilszalicilsav) pyrazolidinek (pl. fenilbutazon) ecetsav származékok (pl. indometacin) fenoxiecetsav származékok (pl. diclofenac, aceclofenac)) oxicamok (pl. piroxicam, meloxicam) propionsav -

WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T (51) International Patent Classification: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A01N 43/00 (2006.01) A61K 31/33 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/US2016/028383 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 20 April 2016 (20.04.2016) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/154,426 29 April 2015 (29.04.2015) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: KARDIATONOS, INC. [US/US]; 4909 DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Lapeer Road, Metamora, Michigan 48455 (US). -

(INN) for Biological and Biotechnological Substances
INN Working Document 05.179 Update 2013 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 2013 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) International Nonproprietary Names (INN) Programme Technologies Standards and Norms (TSN) Regulation of Medicines and other Health Technologies (RHT) Essential Medicines and Health Products (EMP) International Nonproprietary Names (INN) for biological and biotechnological substances (a review) © World Health Organization 2013 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int ) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected] ). Requests for permission to reproduce or translate WHO publications – whether for sale or for non-commercial distribution – should be addressed to WHO Press through the WHO web site (http://www.who.int/about/licensing/copyright_form/en/index.html ). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned.