Emulsions and the HLB System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calcium Stearate Processing
National Organic Standards Board Technical Advisory Panel (TAP) Review Compiled by University of California Sustainable Agriculture Research and Education Program (UC SAREP) for the USDA National Organic Program Calcium Stearate Processing Executive Summary1 A petition is under consideration with respect to NOP regulations subpart G §205.605, governing the use of substances in processed products: Petitioned: Inclusion of calcium stearate on National List of nonagricultural substances allowed in or on processed products labeled as “organic” or “made with organic (specified ingredients or food group(s)).” Calcium stearate is a compound of calcium with a mixture of solid organic acids obtained from edible sources. It is generally used as a solid-phase lubricant that reduces friction between particles of the substance to which it is added. The Petitioner’s intended use is “as a flow agent (anti-dusting agent)” to be used in dry flour based ingredients sold to bakeries (NOSB Petition). The NOP has no prior listing or ruling on the substance. All three reviewers agreed that the substance should be considered synthetic. The reviewers were divided over the use of calcium stearate in food labeled as “organic.” Two of the reviewers felt it should not be allowed in these foods, while one reviewer felt it should be accepted. One reviewer who voted to restrict its use indicated that more information was needed on the nature of the substance and its potential applications, and the other reviewer felt that inclusion of a “synthetic” substance in organics runs contrary to consumer’s expectations regarding organic products. All three reviewers agreed that the substance should be allowed in products labeled as “made with organic…” ingredients. -

Productivity of Castor Oil Plant (Ricinus Communis L.) in the North of Sinaloa
Revista Mexicana Ciencias Agrícolas volume 10 number 5 June 30 - August 13, 2019 Article Productivity of castor oil plant (Ricinus communis L.) in the north of Sinaloa Genny Llaven Valencia1§ Alberto Borbon Gracia2 Xochil Militza Ochoa Espinoza2 Oralia Antuna Grijalva3 Aidé Hernández Hernández3 José Luis Coyac Rodríguez3 1Valle Del Fuerte Experimental Field-INIFAP. Mexico-Nogales International Highway km 1609, Col. Juan José Ríos, Guasave, Sinaloa, Mexico. CP. 81110. 2Experimental Field Norman E. Borlaug-INIFAP. Dr. Norman E. Borlaug Street, km 12, Col. Valle del Yaqui, Cajame, Ciudad Obregón, Sonora. CP. 8500. ([email protected]; [email protected]). 3Universidad Autónoma Agraria Antonio Narro. Periférico Raúl López Sánchez and Santa Fe highway, Torreón, Coahuila, México. CP. 27054. ([email protected]; [email protected]). §Corresponding author: [email protected]. Abstract To determine the productivity of castor oil plant in Sinaloa, the influence of two sowing dates, water availability and two planting densities on the grain yield of four castor oil plant hybrids were evaluated, the trial was established at Valley of Fuerte Experimental Field. During the autumn- winter agricultural cycles with sowing date of December 10, 2015 and spring-summer 2015-2016 with sowing date of February 18, 2016. A randomized complete block design with four replications was used, the plot experimental was four furrows of 20 m long, with a separation of 0.80 m, equivalent to 64 m2, two population densities were managed: 23 000 and 26 000 plants ha-1, and two irrigation regimes per test. The statistical analysis indicated that hybrids 2B-5, Chinatan and HB-8, were higher in yield and without statistical differences, with days at maturity from 145 to 152, so they are considered normal cycle; the average height was 20 m, considered average. -

Essential Wholesale & Labs Carrier Oils Chart
Essential Wholesale & Labs Carrier Oils Chart This chart is based off of the virgin, unrefined versions of each carrier where applicable, depending on our website catalog. The information provided may vary depending on the carrier's source and processing and is meant for educational purposes only. Viscosity Absorbtion Comparible Subsitutions Carrier Oil/Butter Color (at room Odor Details/Attributes Rate (Based on Viscosity & Absorbotion Rate) temperature) Description: Stable vegetable butter with a neutral odor. High content of monounsaturated oleic acid and relatively high content of natural antioxidants. Offers good oxidative stability, excellent Almond Butter White to pale yellow Soft Solid Fat Neutral Odor Average cold weather stability, contains occlusive properties, and can act as a moistening agent. Aloe Butter, Illipe Butter Fatty Acid Compositon: Palmitic, Stearic, Oleic, and Linoleic Description: Made from Aloe Vera and Coconut Oil. Can be used as an emollient and contains antioxidant properties. It's high fluidiy gives it good spreadability, and it can quickly hydrate while Aloe Butter White Soft Semi-Solid Fat Neutral Odor Average being both cooling and soothing. Fatty Acid Almond Butter, Illipe Butter Compostion: Linoleic, Oleic, Palmitic, Stearic Description: Made from by combinging Aloe Vera Powder with quality soybean oil to create a Apricot Kernel Oil, Broccoli Seed Oil, Camellia Seed Oil, Evening Aloe Vera Oil Clear, off-white to yellow Free Flowing Liquid Oil Mild musky odor Fast soothing and nourishing carrier oil. Fatty Acid Primrose Oil, Grapeseed Oil, Meadowfoam Seed Oil, Safflower Compostion: Linoleic, Oleic, Palmitic, Stearic Oil, Strawberry Seed Oil Description: This oil is similar in weight to human sebum, making it extremely nouirshing to the skin. -

Castor Oil Plant (Ricinus Communis)
Invasive plant Castor oil plant Ricinus communis Castor oil plant Ricinus communis Castor oil plant spreads over sandy soil areas, creek banks Also, small amounts of the plant will induce an immunity and gullies. This can lead to a significant loss of prime to poisoning. grazing land. The seeds of castor oil contain ricin, a poison that is Legal requirements extremely toxic to livestock and humans. Leaves have a Castor oil is not a prohibited or restricted invasive lesser amount of toxin. Symptoms of poisoning in animals plant under the Biosecurity Act 2014. However, by law, usually do not appear for a few hours or several days. everyone has a general biosecurity obligation (GBO) to take reasonable and practical steps to minimise the Seeds cause gastrointestinal disorders and leaves tend risks associated with invasive plants under their control. to cause neuromuscular disorders. Poisoning in livestock is rarely reported though, as castor oil plant is seldom grazed by stock when other pasture plants are available. Local governments must have a biosecurity plan that covers lobes; and has small, smooth fruits found in clusters in the invasive plants in their area. This plan may include actions upper parts of the plant. to be taken on certain species. Some of these actions may be required under local laws. Contact your local government Habitat and distribution for more information. Castor oil plant is native to Africa and Asia, and is now Description naturalised throughout Australia. It is often abundant along watercourses and floodplains, disturbed or waste land, and Castor oil plant is a tall, branching perennial shrub that roadsides. -
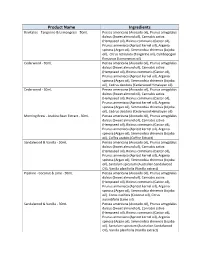
Product Name Ingredients
Product Name Ingredients Revitalise - Tangerine & Lemongrass - 50mL Persea americana (Avocado oil), Prunus amygdalus dulcus (Sweet almond oil), Cannabis sativa (Hempseed oil), Ricinus communis (Castor oil), Prunus armeniaca (Apricot kernel oil), Argania spinosa (Argan oil), Simmondsia chinensis (Jojoba oil), Citrus reticulata (Tangerine oil), Cymbopogon flexuosus (Lemongrass oil) Cedarwood - 30mL Persea americana (Avocado oil), Prunus amygdalus dulcus (Sweet almond oil), Cannabis sativa (Hempseed oil), Ricinus communis (Castor oil), Prunus armeniaca (Apricot kernel oil), Argania spinosa (Argan oil), Simmondsia chinensis (Jojoba oil), Cedrus deodora (Cedarwood Himalayan oil) Cedarwood - 50mL Persea americana (Avocado oil), Prunus amygdalus dulcus (Sweet almond oil), Cannabis sativa (Hempseed oil), Ricinus communis (Castor oil), Prunus armeniaca (Apricot kernel oil), Argania spinosa (Argan oil), Simmondsia chinensis (Jojoba oil), Cedrus deodora (Cedarwood Himalayan oil) Morning Brew - Arabica Bean Extract - 50mL Persea americana (Avocado oil), Prunus amygdalus dulcus (Sweet almond oil), Cannabis sativa (Hempseed oil), Ricinus communis (Castor oil), Prunus armeniaca (Apricot kernel oil), Argania spinosa (Argan oil), Simmondsia chinensis (Jojoba oil), Coffea arabics (Coffee Extract) Sandalwood & Vanilla - 50mL Persea americana (Avocado oil), Prunus amygdalus dulcus (Sweet almond oil), Cannabis sativa (Hempseed oil), Ricinus communis (Castor oil), Prunus armeniaca (Apricot kernel oil), Argania spinosa (Argan oil), Simmondsia chinensis (Jojoba -

Niaproof ® Calcium Stearoyl Lactylate
Technical Data Sheet Ref.: 2019_02v3 Niaproof ® Calcium Stearoyl Lactylate Niaproof® Calcium Stearoyl Lactylate is to exceed 0.5 parts for each 100 parts by offered as a white to cream colored powder weight of flour used. CSL is especially grade. preferred in lean hearth bread type formulations as a dough strengthener. PRODUCT PROPERTIES It is also used as a whipping agent in liquid and frozen egg white at a level not to Chemical Calcium Stearoyl-2- exceed 0.05 percent. In dried egg white at a name Lactylate level not to exceed 0.5 percent. In whipped Formula C48H86CaO12 vegetable oil topping at a level not to exceed 0.3 percent of the weight of the finished White to cream colored whipped vegetable oil topping. Product form powder with a slightly Niaproof CSL is used as a conditioning sweet (caramel) odor agent in dehydrated potatoes in an amount Molecular not to exceed 0.5 percent by weight thereof. 895.27 g/mol weight Legislation CAS No. 5793-94-2 Niaproof Calcium Stearoyl Lactylate EINECS No. 227-335-7 complies with the Food Chemical Codex (FCC). It is a non-toxic additive permitted for HS-code EU 2915.70 direct addition to food for human HS-code US 2915.70 consumption as listed in 21 CFR 172.844. Niaproof CSL is also an approved food Flash point 222 °C additive according to EU with E-number Solubility in Sparingly soluble E482. Water Please check local legislation for the exact dosage levels and allowed applications. Stability Applications Niaproof Calcium Stearoyl Lactylate is stable for 2 years from date of manufacture. -

Alkanoyl Lactyl Lactate Salts As Used in Cosmetics
Safety Assessment of Alkanoyl Lactyl Lactate Salts as Used in Cosmetics Status: Final Report Release Date: July 17, 2019 Panel Date: June 6-7, 2019 The 2019 Cosmetic Ingredient Review Expert Panel members are: Chair, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Ronald A. Hill, Ph.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Executive Director is Bart Heldreth, Ph.D. This report was prepared by Wilbur Johnson, Jr., M.S., Senior Scientific Analyst. © Cosmetic Ingredient Review 1620 L STREET, NW, SUITE 1200 ◊ WASHINGTON, DC 20036-4702 ◊ PH 202.331.0651 ◊ FAX 202.331.0088 ◊ [email protected] ABSTRACT: The Cosmetic Ingredient Review (CIR) Expert Panel (Panel) reviewed the safety of 10 alkanoyl lactyl lactate salts. These ingredients have the surfactant function in cosmetics in common. The Panel reviewed data relevant to the safety of these ingredients, and concluded that these 10 ingredients are safe in cosmetics in the present practices of use and concentration described in the safety assessment when formulated to be non-irritating and non-sensitizing, which may be based on a quantitative risk assessment (QRA) or other accepted methodologies. INTRODUCTION The safety of the following 10 alkanoyl lactyl lactate salts as used in cosmetics is reviewed in this Cosmetic Ingredient Review (CIR) safety assessment. Calcium Stearoyl Lactylate Sodium Cupheoyl Lactylate Sodium Behenoyl Lactylate Sodium Isostearoyl -

Castor Oil Induces Laxation and Uterus Contraction Via Ricinoleic Acid Activating Prostaglandin EP3 Receptors Sorin Tunarua, Till F
Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors Sorin Tunarua, Till F. Althoffa, Rolf M. Nüsingb, Martin Dienerc, and Stefan Offermannsa,d,1 aDepartment of Pharmacology, Max-Planck-Institute for Heart and Lung Research, 61231 Bad Nauheim, Germany; dMedical Faculty, bInstitute for Clinical Pharmacology, J. W. Goethe University Frankfurt, 60590 Frankfurt, Germany; and cInstitute for Veterinary Physiology, Justus Liebig University, 35392 Giessen, Germany Edited by John H. Exton, Vanderbilt University School of Medicine, Nashville, TN, and approved April 25, 2012 (received for review January 30, 2012) Castor oil is one of the oldest drugs. When given orally, it has by the enteric nervous system or are direct effects on intestinal a laxative effect and induces labor in pregnant females. The smooth muscle remained unclear. effects of castor oil are mediated by ricinoleic acid, a hydroxylated The present study was undertaken to elucidate the molecular fatty acid released from castor oil by intestinal lipases. Despite the mechanism underlying the biological effect of castor oil-derived wide-spread use of castor oil in conventional and folk medicine, ricinoleic acid. Based on cellular signaling studies and an siRNA the molecular mechanism by which ricinoleic acid acts remains screening approach, we identified prostaglandin E2 receptors as unknown. Here we show that the EP3 prostanoid receptor is spe- targets of ricinoleic acid and show that the EP3 receptor medi- cifically activated by ricinoleic acid and that it mediates the phar- ates the effects of castor oil on the motility of the uterus and macological effects of castor oil. In mice lacking EP3 receptors, the the intestine. -

Arizona Administrative Code Between the Dates of October 1, 2020 Through December 31, 2020 (Supp
3 A.A.C. 4 Supp. 20-4 December 31, 2020 Title 3 TITLE 3. AGRICULTURE CHAPTER 4. DEPARTMENT OF AGRICULTURE - PLANT SERVICES DIVISION The table of contents on the first page contains quick links to the referenced page numbers in this Chapter. Refer to the notes at the end of a Section to learn about the history of a rule as it was published in the Arizona Administrative Register. Sections, Parts, Exhibits, Tables or Appendices codified in this supplement. The list provided contains quick links to the updated rules. This Chapter contains rule Sections that were filed to be codified in the Arizona Administrative Code between the dates of October 1, 2020 through December 31, 2020 (Supp. 20-4). Table 1. Fee Schedule ......................................................47 Questions about these rules? Contact: Name: Brian McGrew Address: Department of Agriculture 1688 W. Adams St. Phoenix, AZ 85007 Telephone: (602) 542-3228 Fax: (602) 542-1004 E-mail: [email protected] Website: https://agriculture.az.gov/plantsproduce/industrial- hemp-program The release of this Chapter in Supp. 20-4 replaces Supp. 20-3, 1-50 pages Please note that the Chapter you are about to replace may have rules still in effect after the publication date of this supplement. Therefore, all superseded material should be retained in a separate binder and archived for future reference. i PREFACE Under Arizona law, the Department of State, Office of the Secretary of State (Office), accepts state agency rule filings and is the publisher of Arizona rules. The Office of the Secretary of State does not interpret or enforce rules in the Administrative Code. -

Food and Drug Administration, HHS § 172.846
Food and Drug Administration, HHS § 172.846 (i) Polysorbate 65. § 172.844 Calcium stearoyl-2-lactylate. (ii) Polysorbate 60. The food additive calcium stearoyl-2- When used alone, the maximum lactylate may be safely used in or on amount of sorbitan monostearate shall food in accordance with the following not exceed 0.7 percent of the weight of prescribed conditions: the cake icing or cake filling. When (a) The additive, which is a mixture used with polysorbate 65 and/or poly- of calcium salts of stearoyl lactylic sorbate 60, it shall not exceed 0.7 per- acids and minor proportions of other calcium salts of related acids, is manu- cent, nor shall the polysorbate 65 ex- factured by the reaction of stearic acid ceed 0.32 percent or the polysorbate 60 and lactic acid and conversion to the exceed 0.46 percent, and no combina- calcium salts. tion of these emulsifiers shall exceed 1 (b) The additive meets the following percent of the weight of the cake icing specifications: or cake filling. (5) As an emulsifier in solid-state, ed- Acid number, 50–86. Calcium content, 4.2–5.2 percent. ible vegetable fat-water emulsions in- Lactic acid content, 32–38 percent. tended for use as substitutes for milk Ester number, 125–164. or cream in beverage coffee, with or (c) It is used or intended for use as without one or a combination of the follows: following: (1) As a dough conditioner in yeast- (i) Polysorbate 60. leavened bakery products and prepared (ii) Polysorbate 65. mixes for yeast-leavened bakery prod- The maximum amount of the additive ucts in an amount not to exceed 0.5 or additives shall not exceed 0.4 per- part for each 100 parts by weight of cent by weight of the finished edible flour used. -

Formation and Stability of Oil-In-Water Nanoemulsions Containing Rice Bran
Bernardi et al. Journal of Nanobiotechnology 2011, 9:44 http://www.jnanobiotechnology.com/content/9/1/44 RESEARCH Open Access Formation and stability of oil-in-water nanoemulsions containing rice bran oil: in vitro and in vivo assessments Daniela S Bernardi1*, Tatiana A Pereira1, Naira R Maciel1, Josiane Bortoloto1,2, Gisely S Viera1, Gustavo C Oliveira1 and Pedro A Rocha-Filho1 Abstract Background: Nanoemulsions have practical application in a multitude of commercial areas, such as the chemical, pharmaceutical and cosmetic industries. Cosmetic industries use rice bran oil in sunscreen formulations, anti ageing products and in treatments for skin diseases. The aim of this study was to create rice bran oil nanoemulsions using low energy emulsification methods and to evaluate their physical stability, irritation potential and moisturising activity on volunteers with normal and diseased skin types. Results: The nanoemulsion developed by this phase diagram method was composed of 10% rice bran oil, 10% surfactants sorbitan oleate/PEG-30 castor oil, 0.05% antioxidant and 0.50% preservatives formulated in distilled water. The nanoemulsion was stable over the time course of this study. In vitro assays showed that this formulation has a low irritation potential, and when applied to human skin during in vivo studies, the nanoemulsion improved the skin’s moisture and maintained normal skin pH values. Conclusion: The results of irritation potential studies and in vivo assessments indicate that this nanoemulsion has potential to be a useful tool to treat skin diseases, such as atopic dermatitis and psoriasis. dr3n Dc∞γ M Background ω = = k (1) Nanoemulsions are obtained when the size of an emulsion dt ρ2RT globule reaches approximately 20-500 nm. -

Indiana Medical History Museum Guide to the Medicinal Plant Garden
Indiana Medical History Museum Guide to the Medicinal Plant Garden Garden created and maintained by Purdue Master Gardeners of Marion County IMHM Medicinal Plant Garden Plant List – Common Names Trees and Shrubs: Arborvitae, Thuja occidentalis Culver’s root, Veronicastrum virginicum Black haw, Viburnum prunifolium Day lily, Hemerocallis species Catalpa, Catalpa bignonioides Dill, Anethum graveolens Chaste tree, Vitex agnus-castus Elderberry, Sambucus nigra Dogwood, Cornus florida Elecampane, Inula helenium Elderberry, Sambucus nigra European meadowsweet, Queen of the meadow, Ginkgo, Ginkgo biloba Filipendula ulmaria Hawthorn, Crateagus oxycantha Evening primrose, Oenothera biennis Juniper, Juniperus communis False Solomon’s seal, Smilacina racemosa Redbud, Cercis canadensis Fennel, Foeniculum vulgare Sassafras, Sassafras albidum Feverfew, Tanacetum parthenium Spicebush, Lindera benzoin Flax, Linum usitatissimum Witch hazel, Hamamelis virginiana Foxglove, Digitalis species Garlic, Allium sativum Climbing Vines: Golden ragwort, Senecio aureus Grape, Vitis vinifera Goldenrod, Solidago species Hops, Humulus lupulus Horehound, Marrubium vulgare Passion flower, Maypop, Passiflora incarnata Hyssop, Hyssopus officinalis Wild yam, Dioscorea villosa Joe Pye weed, Eupatorium purpureum Ladybells, Adenophora species Herbaceous Plants: Lady’s mantle, Alchemilla vulgaris Alfalfa, Medicago sativa Lavender, Lavendula angustifolia Aloe vera, Aloe barbadensis Lemon balm, Melissa officinalis American skullcap, Scutellaria laterifolia Licorice, Glycyrrhiza