NIWA Client Report No: WLG2012-25 Report Date: May 2013 NIWA Project: CRP12302
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Mollusca, Gastropoda, Conoidea) Dal Pliocene Della Romagna
Quaderno di Studi e Notizie di Storia Naturale della Romagna Quad. Studi Nat. Romagna, 39: 57-61 (giugno 2014) ISSN 1123-6787 Cesare Tabanelli Una nuova specie di Mitromorphidae (Mollusca, Gastropoda, Conoidea) dal Pliocene della Romagna. (Mollusca Gastropoda Conoidea Mitromorphidae) Riassunto Una nuova specie di Mitromorphidae, Mitromorpha scarponii n. sp., è descritta su materiale proveniente da sedimenti del Piacenziano della Romagna. La nuova specie si differenzia dalle congeneri attuali, plioceniche e mioceniche, per la diversa scultura della conchiglia. La miocenica Diptychomitra subovalis Bellardi, 1888 sembra essere la specie più vicina, ma non è stato possibile fare un confronto con il materiale tipo, essendo questo andato perduto. Abstract [A new species of Mitromorphidae (Mollusca, Gastropoda, Conoidea) from the Pliocene of Romagna] A new species of Mitromorphidae, Mitromorpha scarponii n. sp., is described on material from the sediments of Piacenziano of Romagna. The new species differs from congeners, both current and Pliocene and Miocene, for the different sculpture of the shell. Diptychomitra subovalis Bellardi 1888, a Miocene species, appears to be the most similar, but it was not possible to make a comparison with the type material, since it was lost. Key words: Gastropoda, Conoidea, Mitromorpha scarponii n. sp., Pliocene, Romagna, Italy. Premessa TABANELLI & SEGU R INI (1995) fornirono un elenco preliminare dei molluschi pliocenici recuperati dal sito fossilifero di Rio Albonello nei pressi della frazione Marzeno (Brisighella-Ravenna). Successive ricerche portarono al ritrovamento di altre specie fra cui tre reperti di un Mitromorphidae che fu descritto e determinato come Mitromorpha (Mitrolumna) sp. da DELLA BELLA & SC A rp ONI (2007: 53, figg. -

Phylum MOLLUSCA Chitons, Bivalves, Sea Snails, Sea Slugs, Octopus, Squid, Tusk Shell
Phylum MOLLUSCA Chitons, bivalves, sea snails, sea slugs, octopus, squid, tusk shell Bruce Marshall, Steve O’Shea with additional input for squid from Neil Bagley, Peter McMillan, Reyn Naylor, Darren Stevens, Di Tracey Phylum Aplacophora In New Zealand, these are worm-like molluscs found in sandy mud. There is no shell. The tiny MOLLUSCA solenogasters have bristle-like spicules over Chitons, bivalves, sea snails, sea almost the whole body, a groove on the underside of the body, and no gills. The more worm-like slugs, octopus, squid, tusk shells caudofoveates have a groove and fewer spicules but have gills. There are 10 species, 8 undescribed. The mollusca is the second most speciose animal Bivalvia phylum in the sea after Arthropoda. The phylum Clams, mussels, oysters, scallops, etc. The shell is name is taken from the Latin (molluscus, soft), in two halves (valves) connected by a ligament and referring to the soft bodies of these creatures, but hinge and anterior and posterior adductor muscles. most species have some kind of protective shell Gills are well-developed and there is no radula. and hence are called shellfish. Some, like sea There are 680 species, 231 undescribed. slugs, have no shell at all. Most molluscs also have a strap-like ribbon of minute teeth — the Scaphopoda radula — inside the mouth, but this characteristic Tusk shells. The body and head are reduced but Molluscan feature is lacking in clams (bivalves) and there is a foot that is used for burrowing in soft some deep-sea finned octopuses. A significant part sediments. The shell is open at both ends, with of the body is muscular, like the adductor muscles the narrow tip just above the sediment surface for and foot of clams and scallops, the head-foot of respiration. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

The Marine Fauna of New Zealand: the Molluscan Genera Cymatona and Fusitriton (Gastropoda, Family Cymatiidae)
ISSN 0083-7903, 65 (Print) ISSN 2538-1016; 65 (Online) The Marine Fauna of New Zealand: The Molluscan Genera Cymatona and Fusitriton (Gastropoda, Family Cymatiidae) by A. G. BEU New Zealand Oceanographic Institute Memoir 65 1978 NEW ZEALAND DEPARTMENT OF SCIENTIFIC AND INDUSTRIAL RESEARCH The Marine Fauna of New Zealand: The Molluscan Genera Cymatona and Fusitriton (Gastropoda, Family Cymatiidae) by A. G. BEU New Zealand Geological Survey, DSIR, Lower Hutt New Zealand Oceanographic Institute Memoir 65 This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Citation according to ''World List of Scientific Periodicals" (4th edn.): Mem. N.Z. oceanogr. Inst. 65 Received for publication September 1973 © Crown Copyright 1978 This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ CONTENTS Page Abstract . � 5 INTRODUCTION 5 4AXONOMY 10 Family CYMATIIDAE 10 Genus Cymatona 10 Cymatona kampyla 10 Cymatona kampyla kampyla 12 Cymatona kampyla tomlini . 18 Cymatona kampyla jobbernsi 18 Genus Fusitriton 18 Fusitriton cancellatus 22 Fusitriton cancellatus retiolus 22 Fusitriton cance/latus laudandus 23 ECOLOGY . 25 Benthic sampling programme of N.Z. Oceanographic Institute 25 Sampling methods 25 Distribution anomalies 25 Distribution 26 Distribution with depth 26 Distribution with latitude 27 Distribution with sediment type 27 Ecological conclusions 33 Dispersal times and routes of Fusitriton, and their effect on Cymatona 34 Dispersal and distribution 34 Ecological displacement of Cymatona kampyla kampyla 35 ACKNOWLEDGMENTS 36 REFERENCES 36 APPENDIX 1: Station List 38 APPENDIX 2: Dimensions of Cymatona 41 APPENDIX 3: Dimensions of Fusitriton 42 INDEX 44 This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. -
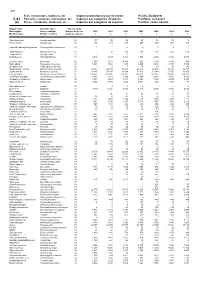
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
478 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southwest C-81 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-ouest (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoccidental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2000 2001 2002 2003 2004 2005 2006 Nombre inglés Nombre científico Grupo de especies t t t t t t t Short-finned eel Anguilla australis 22 37 38 28 27 13 10 5 River eels nei Anguilla spp 22 380 313 337 267 209 277 210 Chinook(=Spring=King)salmon Oncorhynchus tshawytscha 23 1 1 0 4 1 2 1 Sand flounders Rhombosolea spp 31 ... 37 204 193 187 437 514 Tonguefishes Cynoglossidae 31 - - 3 - - - - Flatfishes nei Pleuronectiformes 31 2 954 3 234 2 818 3 308 2 980 3 766 3 050 Common mora Mora moro 32 1 358 1 211 1 308 1 234 1 403 1 154 986 Red codling Pseudophycis bachus 32 5 364 4 526 4 443 8 265 9 540 8 165 5 854 Grenadier cod Tripterophycis gilchristi 32 - 2 7 10 13 13 43 Southern blue whiting Micromesistius australis 32 43 419 49 982 72 203 43 812 26 576 30 304 32 735 Southern hake Merluccius australis 32 16 041 15 188 13 834 22 623 19 344 12 560 12 858 Blue grenadier Macruronus novaezelandiae 32 274 625 247 841 215 302 209 414 147 032 134 145 119 329 Thorntooth grenadier Lepidorhynchus denticulatus 32 3 833 4 783 5 349 5 304 6 341 3 855 4 056 Grenadiers, rattails nei Macrouridae 32 2 394 3 094 3 877 4 253 3 732 2 660 2 848 Gadiformes nei Gadiformes 32 2 853 5 479 3 252 3 281 298 1 217 47 Sea catfishes nei Ariidae 33 .. -

Checklist of Marine Demersal Fishes Captured by the Pair Trawl Fisheries in Southern (RJ-SC) Brazil
Biota Neotropica 19(1): e20170432, 2019 www.scielo.br/bn ISSN 1676-0611 (online edition) Inventory Checklist of marine demersal fishes captured by the pair trawl fisheries in Southern (RJ-SC) Brazil Matheus Marcos Rotundo1,2,3,4 , Evandro Severino-Rodrigues2, Walter Barrella4,5, Miguel Petrere Jun- ior3 & Milena Ramires4,5 1Universidade Santa Cecilia, Acervo Zoológico, R. Oswaldo Cruz, 266, CEP11045-907, Santos, SP, Brasil 2Instituto de Pesca, Programa de Pós-graduação em Aquicultura e Pesca, Santos, SP, Brasil 3Universidade Federal de São Carlos, Programa de Pós-Graduação em Planejamento e Uso de Recursos Renováveis, Rodovia João Leme dos Santos, Km 110, CEP 18052-780, Sorocaba, SP, Brasil 4Universidade Santa Cecília, Programa de Pós-Graduação de Auditoria Ambiental, R. Oswaldo Cruz, 266, CEP11045-907, Santos, SP, Brasil 5Universidade Santa Cecília, Programa de Pós-Graduação em Sustentabilidade de Ecossistemas Costeiros e Marinhos, R. Oswaldo Cruz, 266, CEP11045-907, Santos, SP, Brasil *Corresponding author: Matheus Marcos Rotundo: [email protected] ROTUNDO, M.M., SEVERINO-RODRIGUES, E., BARRELLA, W., PETRERE JUNIOR, M., RAMIRES, M. Checklist of marine demersal fishes captured by the pair trawl fisheries in Southern (RJ-SC) Brazil. Biota Neotropica. 19(1): e20170432. http://dx.doi.org/10.1590/1676-0611-BN-2017-0432 Abstract: Demersal fishery resources are abundant on continental shelves, on the tropical and subtropical coasts, making up a significant part of the marine environment. Marine demersal fishery resources are captured by various fishing methods, often unsustainably, which has led to the depletion of their stocks. In order to inventory the marine demersal ichthyofauna on the Southern Brazilian coast, as well as their conservation status and distribution, this study analyzed the composition and frequency of occurrence of fish captured by pair trawling in 117 fishery fleet landings based in the State of São Paulo between 2005 and 2012. -

Dos Nuevas Especies Cubanas Del Género Mitromorpha Carpenter, 1865 (Mollusca: Neogastropoda: Mitromorphidae)
Avicennia 20: 33-34, 2017 Avicennia © 2017 Avicennia y autores Revista de Biodiversidad Tropical ISNN 1134 - 1785 (www.avicennia.es) Dos nuevas especies cubanas del género Mitromorpha Carpenter, 1865 (Mollusca: Neogastropoda: Mitromorphidae). José Espinosa1 y Jesús Ortea2 1 Instituto de Oceanología, Avda. 1ª nº 18406, E. 184 y 186, Playa, La Habana, Cuba 2 Departamento BOS, Universidad de Oviedo, Asturias, España Resumen: Se describen dos nuevas especies cubanas del género Mitromorpha Carpenter, 1865, recolectadas en la playas de Santa Lucía, Camagüey y Rancho Luna, Cienfuegos, en las regiones nororiental y sur central de Cuba, respectivamente. Abstract: Two Cuban new species of the genus Mitromorpha Carpenter, 1865, from Santa Lucia beach and Rancho Luna beach, in the northeastern and central south of Cuba, respectively, are described.. Mollusca, Neogastropoda, Mitromorphidae, Mitromorpha, new species, Cuba. Key Words: Hasta el presente el género Mitromorpha Carpenter, Descripción: Concha bicónica, subfusiforme alargada y 1865 (familia Mitromorphidae) se encuentra represen- ancha (Id= 2’29), de tamaño pequeño y escultura no can- tado por siete especies en el área antillana: M. biplica- celada (lámina 1A). Protoconcha elevada, formada por ta (Dall, 1889), M. haycocki (Dall & Barstch, 1911), M. una y media vueltas, grandes, salientes y con un núcleo popeae (Faber, 2006), M. erycinella (Espinosa & Ortea, algo señalado. Teleoconcha de cuatro vueltas, moderada- 2008), M. selene (Espinosa & Ortea, 2008), M. cubana mente convexas, la última de las cuales ocupa el 55’3% (Espinosa & Ortea, 2013) y M. bella (Espinosa & Ortea, del largo total de la concha (en vista dorsal). La sutura es 2014); las últimas cuatro descritas descritas las costas estrecha y poco profunda, marcada por un cordón espi- cubanas (véase Dall, 1889; Dall & Barstch, 1911; Faber, ral subsutural, algo crenulado o noduloso por efecto de 2006; Espinosa & Ortea, 2008, 2013 y 2014). -

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean. -

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -

Benthic Field Guide 5.5.Indb
Field Identifi cation Guide to Heard Island and McDonald Islands Benthic Invertebrates Invertebrates Benthic Moore Islands Kirrily and McDonald and Hibberd Ty Island Heard to Guide cation Identifi Field Field Identifi cation Guide to Heard Island and McDonald Islands Benthic Invertebrates A guide for scientifi c observers aboard fi shing vessels Little is known about the deep sea benthic invertebrate diversity in the territory of Heard Island and McDonald Islands (HIMI). In an initiative to help further our understanding, invertebrate surveys over the past seven years have now revealed more than 500 species, many of which are endemic. This is an essential reference guide to these species. Illustrated with hundreds of representative photographs, it includes brief narratives on the biology and ecology of the major taxonomic groups and characteristic features of common species. It is primarily aimed at scientifi c observers, and is intended to be used as both a training tool prior to deployment at-sea, and for use in making accurate identifi cations of invertebrate by catch when operating in the HIMI region. Many of the featured organisms are also found throughout the Indian sector of the Southern Ocean, the guide therefore having national appeal. Ty Hibberd and Kirrily Moore Australian Antarctic Division Fisheries Research and Development Corporation covers2.indd 113 11/8/09 2:55:44 PM Author: Hibberd, Ty. Title: Field identification guide to Heard Island and McDonald Islands benthic invertebrates : a guide for scientific observers aboard fishing vessels / Ty Hibberd, Kirrily Moore. Edition: 1st ed. ISBN: 9781876934156 (pbk.) Notes: Bibliography. Subjects: Benthic animals—Heard Island (Heard and McDonald Islands)--Identification. -

Diet of the Australian Sea Lion (Neophoca Cinerea): an Assessment of Novel DNA-Based and Contemporary Methods to Determine Prey Consumption
Diet of the Australian sea lion (Neophoca cinerea): an assessment of novel DNA-based and contemporary methods to determine prey consumption Kristian John Peters BSc (hons), LaTrobe University, Victoria Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy University of Adelaide (October, 2016) 2 DECLARATION OF ORIGINALITY I certify that this work contains no material which has been accepted for the award of any other degree or diploma in my name, in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. In addition, I certify that no part of this work will, in the future, be used in a submission in my name, for any other degree or diploma in any university or other tertiary institution without the prior approval of the University of Adelaide and where applicable, any partner institution responsible for the joint-award of this degree. I give consent to this copy of my thesis when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. I acknowledge that copyright of published works contained within this thesis resides with the copyright holder(s) of those works. I also give permission for the digital version of my thesis to be made available on the web, via the University’s digital research repository, the Library Search and also through web search engines, unless permission has been granted by the University to restrict access for a period of time.