Dreissena Polymorpha ERSS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Digenetic Trematodes of Marine Teleost Fishes from Biscayne Bay, Florida Robin M
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Parasitology, Harold W. Manter Laboratory of Laboratory of Parasitology 6-26-1969 Digenetic Trematodes of Marine Teleost Fishes from Biscayne Bay, Florida Robin M. Overstreet University of Miami, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Overstreet, Robin M., "Digenetic Trematodes of Marine Teleost Fishes from Biscayne Bay, Florida" (1969). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 867. https://digitalcommons.unl.edu/parasitologyfacpubs/867 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. TULANE STUDIES IN ZOOLOGY AND BOTANY Volume 15, Number 4 June 26, 1969 DIGENETIC TREMATODES OF MARINE TELEOST FISHES FROM BISCAYNE BAY, FLORIDA1 ROBIN M. OVERSTREET2 Institute of Marine Sciences, University of Miami, Miami, Florida CONTENTS ABSTRACT 120 ACKNOWLEDGMENTS ---------------------------------------------------------------------------------------------------- 120 INTRODUCTION -------------------------------------------------------------------------------------------------------------- -

Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Zebra & Quagga Mussels: Species Guide
SPECIES IN DEPTH Zebra and Quagga Mussels NATIVE AND INVASIVE RANGE Zebra mussels are native to the Caspian and Black Seas (Eurasia); quagga mussels are native to the Dneiper River drainage in Ukraine. Both mussels have invaded freshwater systems of the United Kingdom, Western Europe, Canada, and the United States. In the United States, zebra mussels were established in the Great Lakes by 1986; quagga mussels were discovered in the Erie Canal and Lake Ontario in 1991. Now, zebra mussels are widespread in the Great Lakes and all Randy Westbrooks the major river drainages east of the Rocky Mountains. Zebra Mussel Quagga mussels are mostly confined to the lower Great Dreissena polymorpha Lakes area and seem to be replacing zebra mussels where their populations overlap. The zebra mussel is a West Coast distribution freshwater bivalve that, true to its name, is often Quagga and zebra mussels have breached the striped with dark bands Rockies and invaded Western states. Quagga mussels like a zebra, but it can were discovered in Lake Mead (Arizona) in 2007, and Fred Snyder Fred also be pure black or within months their shells were washing up on the unpigmented. This small mussel (about 3 cm shores of Lake Mohave (borders Arizona, California, long) is can be recognized from other mussels by and Nevada), and Lake Havasu (borders California and its triangular shape and one flat edge where its Arizona). From there, the mussels were transported byssal threads (for attaching to hard surfaces; see into many Southern California reservoirs via infested inset photo) emerge. However, it is not as easily Colorado River water that runs through aqueducts distinguished from quagga mussels, as both to the reservoirs, providing the region with half of its have byssal threads, which is a key feature for drinking water. -

Marine Bivalve Molluscs
Marine Bivalve Molluscs Marine Bivalve Molluscs Second Edition Elizabeth Gosling This edition first published 2015 © 2015 by John Wiley & Sons, Ltd First edition published 2003 © Fishing News Books, a division of Blackwell Publishing Registered Office John Wiley & Sons, Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK Editorial Offices 9600 Garsington Road, Oxford, OX4 2DQ, UK The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK 111 River Street, Hoboken, NJ 07030‐5774, USA For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley‐blackwell. The right of the author to be identified as the author of this work has been asserted in accordance with the UK Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. Designations used by companies to distinguish their products are often claimed as trademarks. All brand names and product names used in this book are trade names, service marks, trademarks or registered trademarks of their respective owners. The publisher is not associated with any product or vendor mentioned in this book. Limit of Liability/Disclaimer of Warranty: While the publisher and author(s) have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. -

Silicified Eocene Molluscs from the Lower Murchison District, Southern Carnarvon Basin, Western Australia
[<ecords o{ the Western A uslralian Museum 24: 217--246 (2008). Silicified Eocene molluscs from the Lower Murchison district, Southern Carnarvon Basin, Western Australia Thomas A. Darragh1 and George W. Kendrick2.3 I Department of Invertebrate Palaeontology, Museum Victoria, 1'.0. Box 666, Melbourne, Victoria 3001, Australia. Email: tdarragh(il.Illuseum.vic.gov.au :' Department of Earth and Planetary Sciences, Western Australian Museum, Locked Bag 49, Welshpool D.C., Western Australia 6986, Australia. 1 School of Earth and Ceographical Sciences, The University of Western Australia, 35 Stirling Highway, Crawlev, Western Australia 6009, Australia. Abstract - Silicified Middle to Late Eocene shallow water sandstones outcropping in the Lower Murchison District near Kalbarri township contain a silicified fossil fauna including foraminifera, sponges, bryozoans, solitary corals, brachiopods, echinoids and molluscs. The known molluscan fauna consists of 51 species, comprising 2 cephalopods, 14 bivalves, 1 scaphopod and 34 gastropods. Of these taxa three are newly described, Cerithium lvilya, Zeacolpus bartol1i, and Lyria lamellatoplicata. 25 of these molluscs are identical to or closely comparable with taxa from the southern Australian Eocene. The occurrence of this fauna extends the Southeast Australian Province during the Eocene from southwest Western Australia along the west coast north to at least 27° present day south latitude; consequently the province is here renamed the Southern Australian Province. Keywords: siliceous fossils, Eocene, Kalbarri, molluscs, new taxa, Carnarvon Basin, biogeography, Southern Australian Province. INTRODUCTION The source deposit, a pallid to ferruginous silicified Eocene marine molluscan assemblages from sandstone, forms a weakly defined, low breakaway coastal sedimentary basins in southern Australia trending N-S and sloping gently westward. -

Zebra Mussels Georgia Is Home to Several Species of Mussels, but Thankfully Zebra Mussels Currently Are Not One of Them
Zebra Mussels Georgia is home to several species of mussels, but thankfully zebra mussels currently are not one of them. A small mollusk having “zebra” stripes, it has spread throughout the country and has been described by the USGS as the “poster child of biological invasions”. In areas where it becomes established it can pose significant negative Photo: A Benson - USGS ecological and economic impacts through biofouling and outcompeting native species. Discoveries of Zebra Mussels Zebra Mussel Identification Though zebra mussels (Dreissena polymorpha) have been found in several states since their introduction via ballast water into the Great Lakes, the species has thus far not been found in Georgia. Zebra mussels can be confused with other mussel species, particularly the non-native quagga mussel (Dreissena bugensis). The guide below can help further distinguish the species. Should you have questions regarding identification of a mussel you have found, or if you suspect you have found a zebra mussel, RETAIN THE SPECIMEN and IMMEDIATELY contact your regional Georgia DNR Wildlife Resources Division Fisheries Office. Source: USGS https://nas.er.usgs.gov/queries/factsheet.aspx?speciesid=5 So, What Harm do Zebra Mussels Cause? A notorious mussel introduced into the Great Lakes via ballast water a few decades ago, zebra mussels have since spread to several states throughout the U.S. Though small in size, the mussel has been known to cause enormous-sized problems, most notably its biofouling capabilities that often result in clogged water lines for power plants, industrial facilities, and other commercial entities. Such biofouling has resulted in significant economic costs to several communities. -

Original Papers Species Richness and Diversity of the Parasites of Two Predatory Fish Species – Perch (Perca Fluviatilis Linna
Annals of Parasitology 2015, 61(2), 85–92 Copyright© 2015 Polish Parasitological Society Original papers Species richness and diversity of the parasites of two predatory fish species – perch (Perca fluviatilis Linnaeus, 1758) and zander ( Sander lucioperca Linnaeus, 1758) from the Pomeranian Bay Iwona Bielat, Monika Legierko, Ewa Sobecka Division of Hydrobiology, Ichthyology and Biotechnology of Breeding, Faculty of Food Sciences and Fisheries, West Pomeranian University of Technology, Kazimierza Królewicza 4, 71-550 Szczecin, Poland Corresponding author: Ewa Sobecka; e-mail: [email protected] ABSTRACT. Pomeranian Bay as an ecotone is a transition zone between two different biocenoses, which is characterized by an increase in biodiversity and species density. Therefore, Pomeranian Bay is a destination of finding and reproductive migrations of fish from the rivers entered the area. The aim of the study was to compare parasitic fauna of two predatory fish species from the Pomeranian Bay, collected from the same fishing grounds at the same period. A total of 126 fish studied (53 perches and 73 zanders) were collected in the summer 2013. Parasitological examinations included: skin, fins, gills, vitreous humour and lens of the eye, mouth cavity, body cavity and internal organs. Apart from the prevalence and intensity of infection (mean, range) the parasite communities of both fish species were compared. European perch and zander were infected with parasites from five different taxonomic units. The most numerous parasites were Diplostomum spp. in European perch and Bucephalus polymorphus in zander. The prevalence of infection of European perch ranged from 5.7% ( Diphyllobothrium latum ) to 22.3% ( Diplostomum spp.) and for zander from 1.4% ( Ancyrocephalus paradoxus , Hysterothylacium aduncum ) to 12.3% ( Bucephalus polymorphus ). -
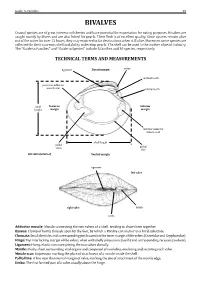
Field Identification Guide to the Living Marine Resources In
Guide to Families 29 BIVALVES Coastal species are of great interest to fisheries and have potential for exportation for eating purposes. Bivalves are caught mainly by divers and are also fished for pearls. Their flesh is of excellent quality. Since oysters remain alive out of the water for over 12 hours, they may exported to far destinations when still alive. Moreover, some species are collected for their nacreous shell and ability to develop pearls. The shell can be used in the mother of pearl industry. The “Guide to Families’’ andTECHNICAL ‘‘Guide to Species’’ TERMS include 5AND families MEASUREMENTS and 10 species, respectively. ligament Dorsal margin umbo posterior adductor cardinal tooth muscle scar lateral tooth Posterior Anterior margin margin shell height anterior adductor muscle scar pallial sinus pallial shell length line left valve (interior) Ventral margin ligament left valve right valve lunule umbo Adductor muscle: Byssus: Chomata: Muscle connecting the two valves of a shell, tending to draw them together. Hinge: Clump of horny threads spun by the foot, by which a Bivalve can anchor to a hard substrate. Ligament: Small denticles and corresponding pits located on the inner margin of the valves (Ostreidae and Gryphaeidae). Mantle: Top interlocking margin of the valves, often with shelly projections (teeth) and corresponding recesses (sockets). Muscle scar: Horny, elastic structure joining the two valves dorsally. Pallial line: Fleshy sheet surrounding vital organs and composed of two lobes, one lining and secreting each valve. Umbo: Impression marking the place of attachment of a muscle inside the shell. A line near the internal margin of valve, marking the site of attachment of the mantle edge. -

Clam Dissection Guideline
Clam Dissection Guideline BACKGROUND: Clams are bivalves, meaning that they have shells consisting of two halves, or valves. The valves are joined at the top, and the adductor muscles on each side hold the shell closed. If the adductor muscles are relaxed, the shell is pulled open by ligaments located on each side of the umbo. The clam's foot is used to dig down into the sand, and a pair of long incurrent and excurrent siphons that extrude from the clam's mantle out the side of the shell reach up to the water above (only the exit points for the siphons are shown). Clams are filter feeders. Water and food particles are drawn in through one siphon to the gills where tiny, hair-like cilia move the water, and the food is caught in mucus on the gills. From there, the food-mucus mixture is transported along a groove to the palps (mouth flaps) which push it into the clam's mouth. The second siphon carries away the water. The gills also draw oxygen from the water flow. The mantle, a thin membrane surrounding the body of the clam, secretes the shell. The oldest part of the clam shell is the umbo, and it is from the hinge area that the clam extends as it grows. I. Purpose: The purpose of this lab is to identify the internal and external structures of a mollusk by dissecting a clam. II. Materials: 2 pairs of safety goggles 1 paper towel 2 pairs of gloves 1 pair of scissors 1 preserved clam 2 pairs of forceps 1 dissecting tray 2 probes III. -

Review and Meta-Analysis of the Environmental Biology and Potential Invasiveness of a Poorly-Studied Cyprinid, the Ide Leuciscus Idus
REVIEWS IN FISHERIES SCIENCE & AQUACULTURE https://doi.org/10.1080/23308249.2020.1822280 REVIEW Review and Meta-Analysis of the Environmental Biology and Potential Invasiveness of a Poorly-Studied Cyprinid, the Ide Leuciscus idus Mehis Rohtlaa,b, Lorenzo Vilizzic, Vladimır Kovacd, David Almeidae, Bernice Brewsterf, J. Robert Brittong, Łukasz Głowackic, Michael J. Godardh,i, Ruth Kirkf, Sarah Nienhuisj, Karin H. Olssonh,k, Jan Simonsenl, Michał E. Skora m, Saulius Stakenas_ n, Ali Serhan Tarkanc,o, Nildeniz Topo, Hugo Verreyckenp, Grzegorz ZieRbac, and Gordon H. Coppc,h,q aEstonian Marine Institute, University of Tartu, Tartu, Estonia; bInstitute of Marine Research, Austevoll Research Station, Storebø, Norway; cDepartment of Ecology and Vertebrate Zoology, Faculty of Biology and Environmental Protection, University of Lodz, Łod z, Poland; dDepartment of Ecology, Faculty of Natural Sciences, Comenius University, Bratislava, Slovakia; eDepartment of Basic Medical Sciences, USP-CEU University, Madrid, Spain; fMolecular Parasitology Laboratory, School of Life Sciences, Pharmacy and Chemistry, Kingston University, Kingston-upon-Thames, Surrey, UK; gDepartment of Life and Environmental Sciences, Bournemouth University, Dorset, UK; hCentre for Environment, Fisheries & Aquaculture Science, Lowestoft, Suffolk, UK; iAECOM, Kitchener, Ontario, Canada; jOntario Ministry of Natural Resources and Forestry, Peterborough, Ontario, Canada; kDepartment of Zoology, Tel Aviv University and Inter-University Institute for Marine Sciences in Eilat, Tel Aviv, -
![Binder 021, Bucephalidae [Trematoda Taxon Notebooks]](https://docslib.b-cdn.net/cover/6980/binder-021-bucephalidae-trematoda-taxon-notebooks-316980.webp)
Binder 021, Bucephalidae [Trematoda Taxon Notebooks]
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Trematoda Taxon Notebooks Parasitology, Harold W. Manter Laboratory of February 2021 Binder 021, Bucephalidae [Trematoda Taxon Notebooks] Harold W. Manter Laboratory of Parasitology Follow this and additional works at: https://digitalcommons.unl.edu/trematoda Part of the Biodiversity Commons, Parasitic Diseases Commons, and the Parasitology Commons Harold W. Manter Laboratory of Parasitology, "Binder 021, Bucephalidae [Trematoda Taxon Notebooks]" (2021). Trematoda Taxon Notebooks. 21. https://digitalcommons.unl.edu/trematoda/21 This Portfolio is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Trematoda Taxon Notebooks by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Family BUCEPHALIDAE POCHE, 1907 1. Bucephalus varicus Manter, 1940 Host. Caranx hippos (Linn.): Common jack; family Carangidae Incidence of Infection. In 1 of 1 host Location. Mainly close to pyloric junction and a few scattered speci mens along length of entire intestine Locality. Bayboro Harbor, Tampa Bay, (new locality record) Florida Discussion. Manter (1940) pictured variation of tentacles and displa- ---- -- cement of organs in preserved B. varicus from the Tropical American Pacific and Tortugas, Florida. We have studied live B. varicus under slight coverslip pressure and can confirm the variations observed by Manter ( 1940) . B. varicus has been reported from no less than eleven different carangid species from Okinawa, the Red Sea, Tortugas, Florida, and the Tropical American Pacific. The only other record of B. varicus from Caranx hippos is by Bravo and Sogandares (1957) from the Pacific Coast of Mexico. -

Bacciger Bacciger (Trematoda: Fellodistomidae) Infection Effects on Wedge Clam Donax Trunculus Condition
Vol. 111: 259–267, 2014 DISEASES OF AQUATIC ORGANISMS Published October 16 doi: 10.3354/dao02769 Dis Aquat Org Bacciger bacciger (Trematoda: Fellodistomidae) infection effects on wedge clam Donax trunculus condition Xavier de Montaudouin1,*, Hocein Bazairi2, Karima Ait Mlik2, Patrice Gonzalez1 1Université de Bordeaux, CNRS, UMR 5805 EPOC, Marine Station, 2 rue du Pr Jolyet, 33120 Arcachon, France 2University Mohammed V - Agdal, Faculty of Sciences, 4 Av. Ibn Battota, Rabat, Morocco ABSTRACT: Wedge clams Donax trunculus inhabit high-energy environments along sandy coasts of the northeastern Atlantic Ocean and the Mediterranean Sea. Two sites were sampled monthly, one in Morocco (Mehdia), where the density was normal, and one in France (Biscarosse), where the density was very low. We tested the hypothesis that the difference in density between the sites was related to infection by the trematode parasite Bacciger bacciger. Identity of both the parasite and the host were verified using anatomical and molecular criteria. Parasite prevalence (i.e. the percentage of parasitized clams) was almost 3 times higher at Biscarosse. At this site, overall prevalence reached 32% in July and was correlated with the migration of several individuals (with a prevalence of 88%) to the sediment surface. After this peak, prevalence decreased rapidly, suggesting death of parasitized clams. The deleterious effect of B. bacciger on wedge clams was also supported by our calculations indicating that the weight of the parasite made up to 56% of the total weight of the parasitized clams. However, condition indices of trematode-free clams were also lower in Biscarosse than in Mehdia or other sites, suggesting that other factors such as pollu- tants or microparasites (Microcytos sp.) may alter wedge clam population fitness in Biscarosse.