Book 2 Part 3 WNV Non-Human Effects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOTE Dimensions and Composition of Mariana Crow Nests on Rota
Micronesica 29(2): 299- 304, 1996 NOTE Dimensionsand Compositionof Mariana Crow Nests on Rota, Mariana Islands MICHAEL R. LUSK 1 AND ESTANISLAO TAISACAN Division of Fish and Wildlife, Rota, MP 96951. Abstract-From 1992 to 1994 we measured dimensions of 11 Mariana crow (Corvus kubaryz) nests on Rota. The mean nest diameter, nest height, inner cup diameter, and cup depth were 37.2 cm, 15.4 cm, 13.3 cm, and 6.9 cm, respectively. These nests consisted of an outer platform and intermediate cup primarily composed Jasminum marianum, and an inner cup mainly of Cocos nucifera frond fibers and Ficus prolixa root lets. The platforms of two nests contained an average of 200 twigs, with most being 2.1-4.0 mm in diameter and 201-250 mm long. The inter mediate cups averaged 93 components, with most twigs measuring 0.0- 2.0 mm in diameter and 101-150 mm long. Total weight of the two nests averaged 347.5 g, with the following breakdown: platform 284.1 g, in termediate cup 42.1 g, and inner cup 21.3 g. Introduction The Mariana crow ( Corvus kubaryz) is the only corvid in Micronesia and occurs only in forested habitats on two islands in the Marianas, Guam and Rota. It was listed as endangered by the U.S. Fish and Wildlife Service in 1984 (U.S. Fish and Wildlife Service 1984). Although basic information is lacking on all aspects of the life history of the Mariana crow, Jenkins (1983), Tomback (1986), and Michael (1987) describe in varying detail Mariana crow nest construction on Guam and Rota. -

Genetic Applications in Avian Conservation
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey 2011 Genetic Applications in Avian Conservation Susan M. Haig U.S. Geological Survey, [email protected] Whitcomb M. Bronaugh Oregon State University Rachel S. Crowhurst Oregon State University Jesse D'Elia U.S. Fish and Wildlife Service Collin A. Eagles-Smith U.S. Geological Survey See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/usgsstaffpub Haig, Susan M.; Bronaugh, Whitcomb M.; Crowhurst, Rachel S.; D'Elia, Jesse; Eagles-Smith, Collin A.; Epps, Clinton W.; Knaus, Brian; Miller, Mark P.; Moses, Michael L.; Oyler-McCance, Sara; Robinson, W. Douglas; and Sidlauskas, Brian, "Genetic Applications in Avian Conservation" (2011). USGS Staff -- Published Research. 668. https://digitalcommons.unl.edu/usgsstaffpub/668 This Article is brought to you for free and open access by the US Geological Survey at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Staff -- Published Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Susan M. Haig, Whitcomb M. Bronaugh, Rachel S. Crowhurst, Jesse D'Elia, Collin A. Eagles-Smith, Clinton W. Epps, Brian Knaus, Mark P. Miller, Michael L. Moses, Sara Oyler-McCance, W. Douglas Robinson, and Brian Sidlauskas This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ usgsstaffpub/668 The Auk 128(2):205–229, 2011 The American Ornithologists’ Union, 2011. Printed in USA. SPECIAL REVIEWS IN ORNITHOLOGY GENETIC APPLICATIONS IN AVIAN CONSERVATION SUSAN M. HAIG,1,6 WHITCOMB M. BRONAUGH,2 RACHEL S. -
![Docket No. FWS–HQ–MB–2018–0047; FXMB 12320900000//201//FF09M29000]](https://docslib.b-cdn.net/cover/7074/docket-no-fws-hq-mb-2018-0047-fxmb-12320900000-201-ff09m29000-1487074.webp)
Docket No. FWS–HQ–MB–2018–0047; FXMB 12320900000//201//FF09M29000]
This document is scheduled to be published in the Federal Register on 04/16/2020 and available online at federalregister.gov/d/2020-06779, and on govinfo.gov Billing Code 4333–15 DEPARTMENT OF THE INTERIOR Fish and Wildlife Service 50 CFR Part 10 [Docket No. FWS–HQ–MB–2018–0047; FXMB 12320900000//201//FF09M29000] RIN 1018–BC67 General Provisions; Revised List of Migratory Birds AGENCY: Fish and Wildlife Service, Interior. ACTION: Final rule. SUMMARY: We, the U.S. Fish and Wildlife Service (Service), revise the List of Migratory Birds protected by the Migratory Bird Treaty Act (MBTA) by both adding and removing species. Reasons for the changes to the list include adding species based on new taxonomy and new evidence of natural occurrence in the United States or U.S. territories, removing species no longer known to occur within the United States or U.S. territories, and changing names to conform to accepted use. The net increase of 67 species (75 added and 8 removed) will bring the total number of species protected by the MBTA to 1,093. We regulate the taking, possession, transportation, sale, purchase, barter, exportation, and importation of migratory birds. An accurate and up-to-date list of species protected by the MBTA is essential for public notification and regulatory purposes. DATES: This rule is effective [INSERT DATE 30 DAYS AFTER DATE OF PUBLICATION IN THE FEDERAL REGISTER]. 1 FOR FURTHER INFORMATION CONTACT: Eric L. Kershner, Chief of the Branch of Conservation, Permits, and Regulations; Division of Migratory Bird Management; U.S. Fish and Wildlife Service; MS: MB; 5275 Leesburg Pike, Falls Church, VA 22041-3803; (703) 358-2376. -
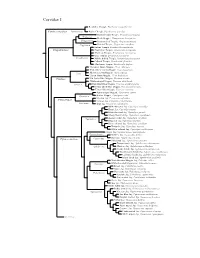
Corvidae Species Tree
Corvidae I Red-billed Chough, Pyrrhocorax pyrrhocorax Pyrrhocoracinae =Pyrrhocorax Alpine Chough, Pyrrhocorax graculus Ratchet-tailed Treepie, Temnurus temnurus Temnurus Black Magpie, Platysmurus leucopterus Platysmurus Racket-tailed Treepie, Crypsirina temia Crypsirina Hooded Treepie, Crypsirina cucullata Rufous Treepie, Dendrocitta vagabunda Crypsirininae ?Sumatran Treepie, Dendrocitta occipitalis ?Bornean Treepie, Dendrocitta cinerascens Gray Treepie, Dendrocitta formosae Dendrocitta ?White-bellied Treepie, Dendrocitta leucogastra Collared Treepie, Dendrocitta frontalis ?Andaman Treepie, Dendrocitta bayleii ?Common Green-Magpie, Cissa chinensis ?Indochinese Green-Magpie, Cissa hypoleuca Cissa ?Bornean Green-Magpie, Cissa jefferyi ?Javan Green-Magpie, Cissa thalassina Cissinae ?Sri Lanka Blue-Magpie, Urocissa ornata ?White-winged Magpie, Urocissa whiteheadi Urocissa Red-billed Blue-Magpie, Urocissa erythroryncha Yellow-billed Blue-Magpie, Urocissa flavirostris Taiwan Blue-Magpie, Urocissa caerulea Azure-winged Magpie, Cyanopica cyanus Cyanopica Iberian Magpie, Cyanopica cooki Siberian Jay, Perisoreus infaustus Perisoreinae Sichuan Jay, Perisoreus internigrans Perisoreus Gray Jay, Perisoreus canadensis White-throated Jay, Cyanolyca mirabilis Dwarf Jay, Cyanolyca nanus Black-throated Jay, Cyanolyca pumilo Silvery-throated Jay, Cyanolyca argentigula Cyanolyca Azure-hooded Jay, Cyanolyca cucullata Beautiful Jay, Cyanolyca pulchra Black-collared Jay, Cyanolyca armillata Turquoise Jay, Cyanolyca turcosa White-collared Jay, Cyanolyca viridicyanus -

Boiga Irregularis (Brown Tree Snakes) on Guam and Its Effect on Fauna
Boiga irregularis (Brown Tree Snakes) on Guam and Its Effect on Fauna Alexandria Amand Introduction The island of Guam, a U. S. Territory, is located in the tropical western Pacific, nearly equidistant from Japan to the north, the Philippines to the west, and New Guinea to the South (Enbring & Ftitts 1988). The island is longer than it is wide and is divided into a diverse landscape with forests and cliffs in the north and primarily savannas and river valleys in the south (Savidge 1987). Guam is also a land of great biodiversity including small mammals, reptiles, and numerous bird species; however, snakes are not a natural part of this biodiversity. The burrowing blind snake (Rhamphotyphlops braminus), the only native snake to Guam, does not pose a threat to the fauna, yet the introduction of Boiga irregularis (brown tree snake) has threatened the island’s biodiversity. Boiga irregularis a native to Indonesia, New Guinea, Solomon Islands, and Australia, was introduced to Guam by way of navy vessels shortly after World War II where it is an exotic and invasive species (Butler 1997). As a result of its accidental introduction and population explosion, Boiga irregularis is responsible for a loss of biodiversity through predation. Boiga irregualris has had devastating effects particularly on avifauna such as the Guam rail and Guam flycatcher, small mammals including the Mariana fruit bat, and reptiles such as geckos and skinks. This paper will outline the negative impact that Boiga irregularis has had on the biodiversity of Guam, as well as the techniques being used to control the snakes population and discuss their effectiveness. -

Adobe PDF, Job 6
Noms français des oiseaux du Monde par la Commission internationale des noms français des oiseaux (CINFO) composée de Pierre DEVILLERS, Henri OUELLET, Édouard BENITO-ESPINAL, Roseline BEUDELS, Roger CRUON, Normand DAVID, Christian ÉRARD, Michel GOSSELIN, Gilles SEUTIN Éd. MultiMondes Inc., Sainte-Foy, Québec & Éd. Chabaud, Bayonne, France, 1993, 1re éd. ISBN 2-87749035-1 & avec le concours de Stéphane POPINET pour les noms anglais, d'après Distribution and Taxonomy of Birds of the World par C. G. SIBLEY & B. L. MONROE Yale University Press, New Haven and London, 1990 ISBN 2-87749035-1 Source : http://perso.club-internet.fr/alfosse/cinfo.htm Nouvelle adresse : http://listoiseauxmonde.multimania. -

Program for 70Th Annual Meeting of the COOPER ORNITHOLOGICAL SOCIETY Riverside, California, 25 - 29 April, 2000
Program and Abstracts Cooper Ornithological Society 70th Annual Meeting 25-29 April 2000 Riverside, California Program for 70th Annual Meeting of the COOPER ORNITHOLOGICAL SOCIETY Riverside, California, 25 - 29 April, 2000 Local Hosts University of California, Riverside Campus University of California Natural Reserve System University of California Cooperative Extension Committee on Local Arrangements John Rotenberry, Chair Tom Scott, Co-chair Paul Aigner Cathy Koehler Karen Bagne Bill Kristan Jutta Burger Michael Patten Sharon Coe Kris Preston Jill Deppe Tim Redman Ian Gillespie Bill Webb Sheila Kee Walter Wehtje Scientific Program Committee Tom Scott, Chair John Rotenberry, Co-chair Charles Collins Kimball Garrett Hugh Ellis Mark Reynolds Steve Zack Sponsors US Geological Survey US Fish and Wildlife Service Region 6 UC Riverside College of Natural and Agricultural Sciences San Bernardino Valley Audubon Society UC Riverside Natural Reserve System UC Cooperative Extension Integrated Hardwood Range Management Program Lynx Edicions Oxford University Press University of Alaska Press Thayer Birding Software UNIVERSITY Habitat Characteristics William D . Berry OF ALASKA of Some Passerine Birds 1954-1956 Alaskan Field Sketches PRESS in Western North American Taiga compiled by Elizabeth Berry • Brina Kessel 1989, paper, reg . $19.95 conf. special $12.00 1998, paper, reg . $16.95 conf, special $13 cloth, reg .95 conf. special $25 To contact us .50 . $39 .00 Over one-third of these eloquent and detailed visit our website: This book describes specific habitat relation- sketches capture a range of Alaska birds . h ttp ://WWW. uaf.ed u/ ships of some of the small land birds that live in the subarctic . It contains descriptions and Accompanied by detailed field notes, the images uapress provide an intimate view of their activities and photographs of typical taiga habitats, color plates of the major bird species discussed, and habitats from hatchlings to adults . -

WILDLIFE in a CHANGING WORLD an Analysis of the 2008 IUCN Red List of Threatened Species™
WILDLIFE IN A CHANGING WORLD An analysis of the 2008 IUCN Red List of Threatened Species™ Edited by Jean-Christophe Vié, Craig Hilton-Taylor and Simon N. Stuart coberta.indd 1 07/07/2009 9:02:47 WILDLIFE IN A CHANGING WORLD An analysis of the 2008 IUCN Red List of Threatened Species™ first_pages.indd I 13/07/2009 11:27:01 first_pages.indd II 13/07/2009 11:27:07 WILDLIFE IN A CHANGING WORLD An analysis of the 2008 IUCN Red List of Threatened Species™ Edited by Jean-Christophe Vié, Craig Hilton-Taylor and Simon N. Stuart first_pages.indd III 13/07/2009 11:27:07 The designation of geographical entities in this book, and the presentation of the material, do not imply the expressions of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily refl ect those of IUCN. This publication has been made possible in part by funding from the French Ministry of Foreign and European Affairs. Published by: IUCN, Gland, Switzerland Red List logo: © 2008 Copyright: © 2009 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Vié, J.-C., Hilton-Taylor, C. -

Warm Temperatures During Cold Season Can Negatively Affect Adult
Warm temperatures during cold season can negatively affect adult survival in an alpine bird Jules Chiffard, Anne Delestrade, Nigel Gilles Yoccoz, Anne Loison, Aurélien Besnard To cite this version: Jules Chiffard, Anne Delestrade, Nigel Gilles Yoccoz, Anne Loison, Aurélien Besnard. Warm tempera- tures during cold season can negatively affect adult survival in an alpine bird. Ecology and Evolution, Wiley Open Access, 2019, 9 (22), pp.12531-12543. 10.1002/ece3.5715. hal-02413017 HAL Id: hal-02413017 https://hal.archives-ouvertes.fr/hal-02413017 Submitted on 7 Jan 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Received: 28 February 2019 | Revised: 5 September 2019 | Accepted: 9 September 2019 DOI: 10.1002/ece3.5715 ORIGINAL RESEARCH Warm temperatures during cold season can negatively affect adult survival in an alpine bird Jules Chiffard1 | Anne Delestrade2,3 | Nigel Gilles Yoccoz2,4 | Anne Loison3 | Aurélien Besnard1 1Ecole Pratique des Hautes Etudes (EPHE), Centre d'Ecologie Fonctionnelle Abstract et Evolutive (CEFE), UMR 5175, Centre Climate seasonality is a predominant constraint on the lifecycles of species in alpine National de la Recherche Scientifique (CNRS), PSL Research University, and polar biomes. -

Breeding Biology and Behaviour of the Forest Raven Corvus Tasmanicus in Southern Tasmania
Breeding Biology and Behaviour of the Forest Raven Corvus tasmanicus in southern Tasmania Clare Lawrence BSc (Hons) Submitted in fulfilment of the requirements for the degree of Master of Science, School of Zoology, University of Tasmania May 2009 This thesis contains no material which has been accepted for a degree of diploma by the University or any other institution. To the best of my knowledge and belief, this thesis contains no material previously published or written by another person, except where due acknowledgement is made in the text. Clare Lawrence Of. o6 Date Statement of Authority of Access This thesis may be available for loan and limited copying in accordance with the Copyright Act 1968 Clare Lawrence 0/. Date TABLE OF CONTENTS TABLE OF CONTENTS 1 ABSTRACT 3 ACKNOWLEDGEMENTS 5 1. GENERAL INTRODUCTION 6 1.1 The breeding biology of birds 6 1.2 The Corvids 11 1.3 The Australian Corvids 11 1.4 Corvus tasmanicus 13 1.4.1 Identification 13 1.4.2 Distribution 13 1.4.3 Life history 15 1.4.4 Interspecific comparisons 16 1.4.5 Previous studies 17 1.5 The Forest Raven in Tasmania 18 1.6 Aims 19 2. BREEDING BIOLOGY OF THE FOREST RAVEN IN SOUTHERN TASMANIA 21 2.1 Introduction 21 2.2 Methods 23 2.2.1 Study sites 23 2.2.2 Nest characteristics 28 2.2.3 Field observations 31 2.2.4 Parental care 33 2.3 Results 37 2.3.1 Nests 37 2.3.2 Nest success and productivity 42 2.3.3 Breeding season • 46 2.3.4 Parental care 52 2.4 Discussion 66 2.4.1 The nest 68 1 2.4.2 Nest success and productivity 78 2.4.3 Breeding season 82 2.4.4 Parental care 88 2.4.5 Limitations of this study 96 2.5 Conclusion 98 3. -

Birds of Conservation Concern 2021 Migratory Bird Program Table of Contents
U.S. Fish & Wildlife Service Birds of Conservation Concern 2021 Migratory Bird Program Table Of Contents Executive Summary 4 Acknowledgments 5 Introduction 6 Methods 7 Geographic Scope 7 Birds Considered 7 Assessing Conservation Status 7 Identifying Birds of Conservation Concern 10 Results and Discussion 11 Literature Cited 13 Figures 15 Figure 1. Map of terrestrial Bird Conservation Regions (BCRs) Marine Bird Conservation Regions (MBCRs) of North America (Bird Studies Canada and NABCI 2014). See Table 2 for BCR and MBCR names. 15 Tables 16 Table 1. Island states, commonwealths, territories and other affiliations of the United States (USA), including the USA territorial sea, contiguous zone and exclusive economic zone considered in the development of the Birds of Conservation Concern 2021. 16 Table 2. Terrestrial Bird Conservation Regions (BCR) and Marine Bird Conservation Regions (MBCR) either wholly or partially within the jurisdiction of the Continental USA, including Alaska, used in the Birds of Conservation Concern 2021. 17 Table 3. Birds of Conservation Concern 2021 in the Continental USA (CON), continental Bird Conservation Regions (BCR), Puerto Rico and Virgin Islands (PRVI), and Hawaii and Pacific Islands (HAPI). Refer to Appendix 1 for scientific names of species, subspecies and populations Breeding (X) and nonbreeding (nb) status are indicated for each geography. Parenthesized names indicate conservation concern only exists for a specific subspecies or population. 18 Table 4. Numbers of taxa of Birds of Conservation Concern 2021 represented on the Continental USA (CON), continental Bird Conservation Region (BCR), Puerto Rico and VirginIslands (PRVI), Hawaii and Pacific Islands (HAPI) lists by general taxonomic groups. Also presented are the unique taxa represented on all lists. -

The State of the Birds 2013 Report on Private Lands United States of America Contents Foreword
The State of the Birds 2013 Report on Private Lands United States of America Contents Foreword .................................. 3 Overview .................................. 4 Wetlands .................................. 6 Grasslands ................................ 10 Aridlands ................................. 14 Forests ................................... 18 Coasts .................................... 28 Islands ................................... 30 Resident Game Birds ........................ 32 Opportunities for Improving Private Lands Conservation .............................. 36 Urban and Suburban Landscapes .............. 40 Private Lands Conservation Programs ........... 41 Our Approach .............................. 44 Cover photos (clockwise from top left): Wood Duck pair by Roy Lammle; Sage- Grouse and Cows by Jane Elliot; Red Knot by Greg Gard; Grass photo by Jason Johnson, USDA-NRCS, Iowa; Henslow's Sparrow by Joshua Clark, www. momentsinature.com This page: Montana rancher Dennis Mercer, by Conservation Media for Sage Grouse Initiative; Dickcissel by Phil Hauck; www.flickr.com/photos/ dharma_for_one 2 FOREWORD “When land does well for its owner, and the owner does well by his land; when both end up better by reason of their partnership, we have conservation.” —Aldo Leopold, The Farmer as a Conservationist Legendary conservationist Aldo Leopold began his career in the U.S. Forest Service in the Southwest, where he learned about resource management on public lands. But as he returned to the Midwest where he was raised—and