Community Structure of the Harpacticoida
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thiruvallur District
DISTRICT DISASTER MANAGEMENT PLAN FOR 2017 TIRUVALLUR DISTRICT tmt.E.sundaravalli, I.A.S., DISTRICT COLLECTOR TIRUVALLUR DISTRICT TAMIL NADU 2 COLLECTORATE, TIRUVALLUR 3 tiruvallur district 4 DISTRICT DISASTER MANAGEMENT PLAN TIRUVALLUR DISTRICT - 2017 INDEX Sl. DETAILS No PAGE NO. 1 List of abbreviations present in the plan 5-6 2 Introduction 7-13 3 District Profile 14-21 4 Disaster Management Goals (2017-2030) 22-28 Hazard, Risk and Vulnerability analysis with sample maps & link to 5 29-68 all vulnerable maps 6 Institutional Machanism 69-74 7 Preparedness 75-78 Prevention & Mitigation Plan (2015-2030) 8 (What Major & Minor Disaster will be addressed through mitigation 79-108 measures) Response Plan - Including Incident Response System (Covering 9 109-112 Rescue, Evacuation and Relief) 10 Recovery and Reconstruction Plan 113-124 11 Mainstreaming of Disaster Management in Developmental Plans 125-147 12 Community & other Stakeholder participation 148-156 Linkages / Co-oridnation with other agencies for Disaster 13 157-165 Management 14 Budget and Other Financial allocation - Outlays of major schemes 166-169 15 Monitoring and Evaluation 170-198 Risk Communications Strategies (Telecommunication /VHF/ Media 16 199 / CDRRP etc.,) Important contact Numbers and provision for link to detailed 17 200-267 information 18 Dos and Don’ts during all possible Hazards including Heat Wave 268-278 19 Important G.Os 279-320 20 Linkages with IDRN 321 21 Specific issues on various Vulnerable Groups have been addressed 322-324 22 Mock Drill Schedules 325-336 -

106Th MEETING
106th MEETING TAMIL NADU STATE COASTAL ZONE MANAGEMENT AUTHORITY Date: 25.07.2019 Venue: Time: 11.00 A.M Conference Hall, 2nd floor, Namakkal Kavinger Maligai, Secretariat, Chennai – 600 009 INDEX Agenda Pg. Description No. No. 01 Confirmation of the minutes of the 105th meeting of the Tamil Nadu State 1 Coastal Zone Management Authority held on 21.05.2019 02 The action taken on the decisions of 105th meeting of the Authority held on 12 21.05.2019 03 Construction of 30” OD Underground Natural Gas Pipeline of M/s. Indian Oil Corporation Ltd., from Ennore LNG Terminal situated inside Kamarajar Port Limited, Ennore, Tiruvallur district to Salavakkam Village, Uthiramerur Taluk, 15 Kancheepuram district 04 Construction of doubling of Railway Line between Existing Holding Yard No.1 at Ch.00m (Near Bridge No.5) to Entry of Container Rail Terminal Yard of M/s. Kamarajar Port Ltd., at Athipattu, Puzhuthivakkam and Ennore Village of 17 Ponneri Taluk, Tiruvallur district 05 Erection of Transmission tower and transmission line for 400 KV power evacuation line from SEZ to Ennore Thermal Power Station (ETPS) expansion project, SEZ to North Chennai (NC) Pooling Station, EPS expansion project to NC Pooling Station and 765 KV Power evacuation line from North Chennai 19 Thermal Power Station-Stage-III (NCTPS-III) to NC Pooling Station at Ennore by M/s. Tamil Nadu Transmission Corporation Limited (TANTRANSCO) 06 Revalidation of CRZ Clearance for the Foreshore facilities viz., Pipe Coal Conveyor, Cooling Water Intake and Outfall Pipeline for the project and ETPS Expansion Thermal Power Project (1x660 MW) proposed within the existing 21 ETPS at Ernavur Village, Thiruvottiyur Taluk, Tiruvallur district proposed by TANGEDCO 07 Proposed Container Transit Terminal at S.F.No.1/3B3, Pulicat Road, Kattupalli Village, Tiruvallur district by M/s. -

Advance Tour Programme for Division Fever Camps 17.04.2021
Advance Tour Programme for Division Fever Camps 17.04.2021 FIRST CAMP LOCATION SECOND CAMP LOCATION THIRD CAMP LOCATION SNO ZONE DIVISION MO POSTED 8.30 AM TO 11 AM 11.30 AM TO 1.30 PM 4.00 AM TO 7.00 PM 1 4 Magaliamman Kovil street , Ernavoor , Perumal kovil street , Ernavoor , Chennai Dr.Prasath 2 2 Thilagar Nagar , Ennore , Chennai 57 Kamraj Nagar , , Ennore , Chennai 57 Dr.Arun Vivekanandar 3 11 Kailash Sector , Thiruvottiyur , Chennai 19 Janakiyammal Estate , Thiruvottiyur , Dr.Arunya Thiruvottiyur 4 12 & 13 Sathangadu High Road , ( Near St.Paul's School Ayyapillaithottam 2nd Street , Dr.Vijiyalakshmi 5 9 Thiruvottiyur High road , Thiruvottiyur , Pattinathar Kovil street , , Thiruvottiyur , Dr.Vinodh 6 8 Masthan kovil Street , Thiruvottiyur Dr.Saranyavathi 7 19 1st Main road , Mathur, Manali 3rd main road, Mathur, Manali . Dr.Benith Manali 8 18 Thillaipuram, Manali Div.20. New MGR Nagar, Manali Dr. Elumalai 9 22 GANDHI MAIN STREET DR. RAMESH RAJA 10 24 SIVA PRAKASAM NAGAR DR. RAMESH RAJA 11 25 KATTIDA THOLLILALAR NAGAR DR. CHRISTINA 12 26 THIRUMALAI NAGAR SASTHRI NAGAR 1. DR. MADUBALA 13 27 IDAIMA NAGAR DR. PRAVEEN KUMAR 14 Madhavaram 28 ARINGAR ANNA STREET DR. NIRMAL KUMAR 15 29 DEVARAJAN STREET DR. SATHYA DEVI 16 30 KAMARAJ STREET DR. PAUL JAISON 17 31 MUTHUMARIAMMAN KOIL STREET DR. ACHINA TITUS 18 32 SARATHY NAGAR 1ST STREET DR. VIDHYA 19 33 KAMARAJ SALAI DR. MYTHILI 20 34 R V NAGAR SOLAIAMMAN KOIL STREET DR. STEPHEN 21 35 T H ROAD CHINNADIMADAM MUTHAMIL NAGAR 7TH BLOCK ICDS Dr.Mathina, 22 36 MKB NAGAR BUS DEPO INDUSTRIAL ESTATE Dr.Thahaseen 23 37 MULLAI COMPLEX VALLUVAR STREET Dr.Joseph 24 38 NETHAJI NAGAR MAIN ROAD NETHAJI NAGAR 5TH ST Dr.Karan 25 39 A E SCHEME ROAD NAGOORAR THOTTAM Dr.Thirumalai raj 26 40 CORPORATION COLONY T SUNAMI QUATETRS Dr.kalaiyarasi 27 Tondiarpet 41 ANNA NAGAR DEVIKARUMARIAMMAN NAGAR DR.Zoharath 28 42 T H ROAD MAIN ST THILAGAR NAGAR COMMUNITY HALL DR. -

The Institute of Road Transport Driver Training Wing, Gummidipundi
THE INSTITUTE OF ROAD TRANSPORT DRIVER TRAINING WING, GUMMIDIPUNDI LIST OF TRAINEES COMPLETED THE HVDT COURSE Roll.No:17SKGU2210 Thiru.BARATH KUMAR E S/o. Thiru.ELANCHEZHIAN D 2/829, RAILWAY STATION ST PERUMAL NAICKEN PALAYAM 1 8903739190 GUMMIDIPUNDI MELPATTAMBAKKAM PO,PANRUTTI TK CUDDALORE DIST Pincode:607104 Roll.No:17SKGU3031 Thiru.BHARATH KUMAR P S/o. Thiru.PONNURENGAM 950 44TH BLOCK 2 SATHIYAMOORTHI NAGAR 9789826462 GUMMIDIPUNDI VYASARPADI CHENNAI Pincode:600039 Roll.No:17SKGU4002 Thiru.ANANDH B S/o. Thiru.BALASUBRAMANIAN K 2/157 NATESAN NAGAR 3 3RD STREET 9445516645 GUMMIDIPUNDI IYYPANTHANGAL CHENNAI Pincode:600056 Roll.No:17SKGU4004 Thiru.BHARATHI VELU C S/o. Thiru.CHELLAN 286 VELAPAKKAM VILLAGE 4 PERIYAPALAYAM PO 9789781793 GUMMIDIPUNDI UTHUKOTTAI TK THIRUVALLUR DIST Pincode:601102 Roll.No:17SKGU4006 Thiru.ILAMPARITHI P S/o. Thiru.PARTHIBAN A 133 BLA MURUGAN TEMPLE ST 5 ELAPAKKAM VILLAGE & POST 9952053996 GUMMIDIPUNDI MADURANDAGAM TK KANCHIPURAM DT Pincode:603201 Roll.No:17SKGU4008 Thiru.ANANTH P S/o. Thiru.PANNEER SELVAM S 10/191 CANAL BANK ROAD 6 KASTHURIBAI NAGAR 9940056339 GUMMIDIPUNDI ADYAR CHENNAI Pincode:600020 Roll.No:17SKGU4010 Thiru.VIJAYAKUMAR R S/o. Thiru.RAJENDIRAN TELUGU COLONY ROAD 7 DEENADAYALAN NAGAR 9790303527 GUMMIDIPUNDI KAVARAPETTAI THIRUVALLUR DIST Pincode:601206 Roll.No:17SKGU4011 Thiru.ULIS GRANT P S/o. Thiru.PANNEER G 68 THAYUMAN CHETTY STREET 8 PONNERI 9791745741 GUMMIDIPUNDI THIRUVALLUR THIRUVALLUR DIST Pincode:601204 Roll.No:17SKGU4012 Thiru.BALAMURUGAN S S/o. Thiru.SUNDARRAJAN N 23A,EGAMBARAPURAM ST 9 BIG KANCHEEPURAM 9698307081 GUMMIDIPUNDI KANCHEEPURAM DIST Pincode:631502 Roll.No:17SKGU4014 Thiru.SARANRAJ M S/o. Thiru.MUNUSAMY K 5 VOC STREET 10 DR. -
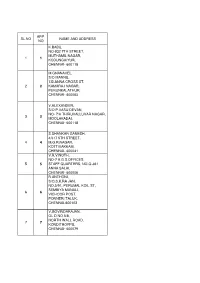
Sl.No App. No Name and Address 1 1 K.Babu, No-932
APP. SL.NO NAME AND ADDRESS NO K.BABU, NO-932 7TH STREET, MUTHAMIL NAGAR, 1 1 KODUNGAIYUR, CHENNAI- 600118 M.GNANAVEL, S/O MANNU, 7.B.ANNA CROSS ST, 2 2 KAMARAJ NAGAR, PERUNKALATHUR, CHENNAI- 600063 V.ALEXANDER, S/O P.VASU DEVAN, NO- 7/A THIRUVALLUVAR NAGAR, 3 3 MOOLAKADAI, CHENNAI- 600118 S.SHANKAR GANESH, 4/317 5TH STREET, 4 4 M.G.R.NAGAR, KOTTIVAKKAM, CHENNAI- 600041 V.R.VINOTH, NO-7 A.G.S.OFFICES, 5 5 STAFF QUARTERS, NO-Q-361 ANNA SALAI, CHENNAI- 600006 R.ANTHONI, S/O.S.K.RA JAN, NO.5/91, PERUMAL KOIL ST, SEMBIYA MANALI, 6 6 VICHOOR POST, PONNERI TALUK, CHENNAI-600103 V.GOVINDARAJAN, OL D NO.5/8, NORTH WALL ROAD, 7 7 KONDITHOPPU, CHENNAI- 600079 G.SIVAKUMAR, NO- 39/12 GANGAIAMMAN KOIL ST, 8 8 LAKSHMIPURAM, THIRUVANMIYUR CHENNAI- 600041 D.MOHAN, N0.22,KARUNANITHI ST, 9 9 KODUNGAYUR, CHENNAI- 600118 G.KARTHIKEYAN, 56,III RD BLOCK, HOUSING BOARD, 10 10 SATHYAMURTY NAGAR, VYASARPADI, CHENNAI-600039 C.SRINIVASAN, 1, 88TH SETREET, 11 11 ASHOK NAGAR, CHENNAI-600083 S,SIVASUBRAMANI, NO.137,5-BLOCK, 4THFLOOR, 12 12 HOUSING BOARD, PERIYAR NAGAR, PULIANTHOPE, CHENNAI- 600012 N.SATHISH, 13 13 NO.27, RADAS NAGAR, CHENNAI- 600021 D.SHANMUGAM, 69/37, ANGALAMMAN KOIL ST, 14 14 GOVINDAPURAM, CHENNAI- 600012 V. MUNIRAJ, 59, SOLAIAMMAN ST,, KODUNGAIYUR, 15 15 CHENNAI- 600118 C.KARNAN, N.NO.24,ARULAYAMMANPET, 16 16 GUINDY CHENNAI-600032 K.KARTHICK, NO.9,PER IYA PALAYATHAMAN KOIL , 17 17 7TH ST, MOOLAKOTHALAM, CHENNAI- 600021 DILLIBABU M, NO.5, ELUMALAI ST, 18 18 SAIDAPET, CHENNAI- 600015 R.MURUGAN, NEW NO.172,OLD NO.203, DOSS NAGAR, 19 19 5TH STREET, -

III Typewriting Institutions Renewal Approvel-2021
DIRECTORATE OF TECHNICAL EDUCATION, CHENNAI-600 025 Phase - III Typewriting Institutions Renewal Approval - 2021 EXTENSION OF SL. APPROVAL COURSE APPROVED FOR INSTITUTE NAME INSTITUTE ADDRESS PROPRIETOR NAME APPROVAL GIVEN NO. NO. CONDUCTING CLASSES UPTO MANI'S INSTITITUE OF OLD NO. 273, NEW NO.463, 1ST FLOOR, P.H. 1 201023 SATHYA MURTHY V 1, 2, 11, 12, 21, 22, 5, 25, 3, 13, 23 2021 COMMERCE ROAD, AMINJIKARAI CHENNAI - 600029 OLD NO 266, NEW NO 449, POONAMALLEE THE MADRAS COLLEGE 2 201090 HIGH ROAD, MADURAVOYAL CHENNAI - ABITHAKUJAMBAL N 1, 2, 11, 12, 21, 22, 5, 25, 3, 13, 23 2021 OF COMMERCE 600029 KAMALA COMMERCIAL NO.130, KAMARAJ NAGAR, ERNAVOOR 3 201117 KATHIRESAN K 1, 2, 11, 12, 21, 22 2021 INSTITUTE CHENNAI - 600057 PARAMOUNT 2181, TNHB, AYAPPAKAM, CHENNAI - 4 201184 S SUBA 1, 2, 11, 12, 21, 22 2021 COMMERCIAL INSTITUTE 600077 THE PERIYAR NAGAR C 409, 4TH STREET, PERIYAR NAGAR, 5 201205 INSTITUTE OF JAYAKUMAR A 1, 2, 11, 12, 21, 22 2021 CHENNAI - 600082 COMMERCE NO. 1/328A, 2ND STREET, MASILAMANI AMALI TYPEWRITING 6 201208 NAGAR, MAATHUR, MADHAVARAM POST, VIJAYALAKSHMI P 1, 2, 11, 12, 21, 22 2021 INSTITUTE CHENNAI - 600060 RAJAN TYPEWRITING 198/6 ASIADD COLONY, ANNA NAGAR WEST 7 201244 VASANTHARA R 1, 2, 11, 12, 21, 22 2021 INSTITUTE CHENNAI - 600101 GOWRI INSTITUTE OF NO.42/4, BALAJI NAGAR MAIN ROAD, 8 201282 BHUVANESHWARI S 1, 2, 11, 12, 21, 22, 3, 13, 23 2021 COMMERCE KOLATHUR, CHENNAI - 600099 BANU TECHNICAL NO.5/16, GANDHI MATHI ST, VETTRI NAGAR, 9 201297 M.MOHAN 1, 2, 11, 12, 21, 22 2021 INSTITUTE CHENNAI - 600082 OLD NO.121, NEW NO.128, 4 TH CROSS ASHWIN COMMERCIAL 10 201348 STREET, SRINIVASA NAGAR, KOLATHUR, MURALIDHARAN M 1, 2, 11, 12, 21, 22, 3 13, 23 2021 INSTITUTE CHENNAI - 600099 NO.27, MADURAIVEERAN STREET, JEEVAN SRI RAM COLLEGE OF 11 201363 BEEMA NAGAR, ANNA NAGAR, WEST EXTN. -
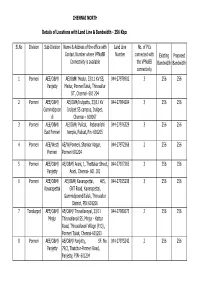
CHENNAI NORTH Sl.No Division Sub-Division Name & Address Of
CHENNAI NORTH Details of Locations with Land Line & Bandwidth - 256 Kbps Sl.No Division Sub-Division Name & Address of the office with Land Line No. of PCs Contact Number where VPNoBB Number connected with Existing Proposed Connectivity is available the VPNoBB Bandwidth Bandwidth connectivity 1 Ponneri AEE/O&M/ AE/O&M/ Medur, 33/11 KV SS, 044-27978902 3 256 256 Panjetty Medur, PonneriTaluk, Thiruvallur DT, Chennai- 601 204 2 Ponneri AEE/O&M/ AE/O&M/Irulipattu, 33/11 KV 044-27984204 3 256 256 Gummidipoon Irulipet SS campus, Irulipet, di Chennai – 600067 3 Ponneri AEE/O&M/ AE/O&M/ Pulicat, Pabanarishi 044-27976329 3 256 256 East Ponneri temple, Pulicat, Pin -601205 4 Ponneri AEE/West/ AE/W/Ponneri, Shankar Nagar, 044-27972368 2 256 256 Ponneri Ponneri 601204 5 Ponneri AEE/O&M/ AE/O&M/ Arani, 1, Thottakar Street, 044-27927265 3 256 256 Panjetty Arani, Chennai- 601 101 6 Ponneri AEE/O&M/ AE/O&M/ Kavarapettai, 465, 044-27925238 3 256 256 Kavarapettai GNT Road, Kavarapettai, GummidipoondiTaluk, Thiruvallur District, PIN 601206 7 Tondiarpet AEE/O&M/ AE/O&M/ Tiruvellavoyal, 33/11 044-27980675 2 256 256 Minjur Thiruvallavoil SS, Minjur - Kattur Road, Thiruvallavoil Village (P.O) , Ponneri Taluk, Chennai-601203 8 Ponneri AEE/O&M/ AE/O&M/ Panjetty, SF. No. 044-27975242 2 256 256 Panjetty 79/2, Thatchur-Ponneri Road, Panjetty, PIN- 601204 Details of Locations with Land Line & Bandwidth - 512 Kbps Sl.No Division Sub-Division Name & Address of the office with Land Line No. of PCs Contact Number where VPNoBB Number connected with Existing Proposed Connectivity is available the VPNoBB Bandwidth Bandwidth connectivity 1 T.Nagar Teynampet DMS SS, DMS Complex, Anna Salai, 044-24332950 3 512 512 Chennai – 6 2 T.Nagar Saidapet Thodunter Nagar SS, No.22, 044-24322211 3 512 512 Thodunter Nagar, Saidapet, CH- 15 3 T.Nagar Saidapet MHU SS, No.1 Link Road, MHU 044-24363191 1 512 512 Compound, CIT Nagar, Ch-35. -
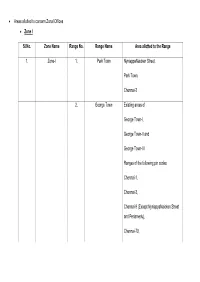
• Areas Allotted to Concern Zonal Offices • Zone I Sl.No. Zone Name Range No. Range Name Area Allotted to the Range 1. Zone
• Areas allotted to concern Zonal Offices • Zone I Sl.No. Zone Name Range No. Range Name Area allotted to the Range 1. Zone-I 1. Park Town NyniappaNaicken Street, Park Town, Chennai-3 2. George Town Existing areas of George Town-I, George Town-II and George Town-III Ranges of the following pin codes: Chennai-1, Chennai-3, Chennai-9 (Except NyniappaNaicken Street and Periamedu), Chennai-79, Chennai-108. 3. Tondiarpet-I Existing Tondiarpet-I areas of Chennai Corporation. 4. Tondiarpet-II Existing Tondiarpet-II areas of Chennai Corporation. 5. Egmore Chennai Corporation postal pin code: Chennai-8, Chennai-34 and Periamedu, Chennai-3. 6. Veppery Chennai Corporation postal pin code: Chennai-7, Chennai-112. 7. Perambur Chennai Corporation postal pin code: Chennai-11, Chennai-12. 8. Vysarpadi Chennai Corporation postal pin code: Chennai-118, Chennai-39 and Chennai-51. Zone-II Sl.No. Zone Name Range No. Range Name Area allotted to the Range 2. Zone-II 9. Arumbakkam Chennai Corporation postal pin code: Arumbakkam, Chennai-106, Ammjikarai, Chennai-29, Koyembedu, Chennai-107. 10. Anna Nagar Chennai Corporation postal pin code: Anna Nagar, Chennai-4, Shenoy Nagar, Chennai-30, Anna Nagar West Extn., Chennai-101. Of Pre-extended Chennai Corporation (of Chennai Revenue District). 11. Ayanavaram Chennai Corporation postal pin code: Ayanavaram, Chennai-23, Periyar Nagar, Chennai-82. 12. Villivakkam Chennai Corporation postal pin code: Villivakkam, Chennai-49, Kolathur, Chennai-99, Anna Nagar East, Chennai-102. 13. Kilpauk Chennai Corporation postal pin code: Kilpauk, Chennai-10, Flowers Road, Purasawakkam.. 14. Kodambakkam Chennai Corporation postal pin code: Kodambakkam, Chennai-24, Choolaimedu, Chennai-94. -
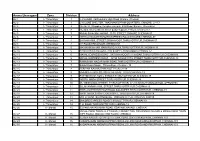
Untitled Spreadsheet
Amma Unavagam Zone Division Address AU 1 I - Thiruvottyur 1 J.J.NAGAR, Kathivakkam High Road ,Ennore ,Chennai AU 2 I - Thiruvottyur 1 CYCLONE SHELTER, THAZHANKUPPAM QUARTERS, ENNORE, CH-57 AU 3 I - Thiruvottyur 2 No.267-A, Shopping Complex campus, K.H Road, Ennore, Chennai-57. AU 4 I - Thiruvottyur 2 No.46/4, Grama Street, Sivan padai kuppam, Ennore, Chennai-57. AU 5 I - Thiruvottyur 3 ANNAI SIVAKAMI NAGAR, 10 TH STREET,ENNORE,CHENNAI-57 AU 6 I - Thiruvottyur 3 NETHAJI NAGAR ENNORE,EXPRESS ROAD,ENNORE,CHENNAI-57 AU 7 I - Thiruvottyur 4 BAJANAI KOIL STREET,ERANAVOOR,THIRUVOTTIYUR,CHENNAI-57 AU 8 I - Thiruvottyur 4 KH ROAD,ERNAVOOR,CHENNAI-57 AU 9 I - Thiruvottyur 5 MANIKAM NAGAR MAIN ROAD,AJAX,THIRUVOTTRIYUR,CHENNAI-19 AU 10 I - Thiruvottyur 5 BHARATHIYAR NAGAR, 2ND STREET,ERNAVOOR,CHENNAI-57 AU 11 I - Thiruvottyur 6 MANALI EXPRESS ROAD , SATHYA MOORTHY NAGAR.THIRUVOTTRIYUR,CHENNAI19 AU 12 I - Thiruvottyur 6 MANALI EXPRESS ROAD , JOTHI NAGAR 10TH STREET.THIRUVOTTIYUR,CHENNAI-19 AU 13 I - Thiruvottyur 7 RAMASAMY NAGAR MAIN ROAD,THIRUVOTTRIYUR,CHENNAI-19 AU 14 I - Thiruvottyur 7 Balakrishana Nagar , Thiruvottiyur, Chennai - 19 AU 15 I - Thiruvottyur 8 PERIYAR NAGAR,THIRUVOTTIYUR,CH-19 AU 16 I - Thiruvottyur 8 ENNORE EXPRESS ROAD.THIRUVOTTIYUR,CHENNAI-19 AU 17 I - Thiruvottyur 9 PATTINATHAR KOVIL STREET,THIRUVOTTRIYUR,CHENNAI-19 AU 18 I - Thiruvottyur 10 NORTH MADA STREET,THIRUVOTTIYUR,CHENNAI-19 AU 19 I - Thiruvottyur 10 55, WESTMADA STREET, THIRUVOTTIYUR, CH-19 (MUKUTHAMMAN KOIL OPPOSITE) AU 20 I - Thiruvottyur 11 ELLAIYAMMAN KOIL STREET,THIRUVOTTRIYUR,CHENNAI-19 AU 21 I - Thiruvottyur 11 THIRUVOTTRIYUR HIGH ROAD,KALADIPET,THIRUVOTTRIYUR, CHENNAI-19 AU 22 I - Thiruvottyur 12 WEST MADA STREET,KALADIPET, TIRUVOTTRIYUR,CHENNAI-19 AU 23 I - Thiruvottyur 13 M G R ROAD, SATHANGADU, THIRUVOTTIYUR, CHENNAI-600019 AU 24 I - Thiruvottyur 14 ENNORE EXPRESS ROAD,THIRUVOTTRIYUR,CHENNAI-19. -

Tiruvottiyur Municipality - Administrative Status
Tamil Nadu Urban Infrastructure Financial Services Limited City Corporate Plan cum Business Plan for Tiruvottriyur Municipality Final Report March 2008 ICRA Management Consulting Services Limited Contents 1. INTRODUCTION ............................................................................................................................................ 1 1.1 BACKGROUND TO THE STUDY ..................................................................................................................... 1 1.2 OBJECTIVES, SCOPE OF WORK AND STUDY MODULES .................................................................................. 1 1.3 APPROACH AND METHODOLOGY ................................................................................................................ 2 1.4 ORGANIZATION OF THIS REPORT ................................................................................................................. 2 2. TOWN PROFILE AND CITY DEMOGRAPHICS ........................................................................................... 3 2.1 LOCATION ................................................................................................................................................ 3 2.2 TIRUVOTTIYUR MUNICIPALITY - ADMINISTRATIVE STATUS ............................................................................... 3 2.3 POPULATION ............................................................................................................................................ 1 2.4 POPULATION PROJECTIONS....................................................................................................................... -

Integrated Urban Flood Management for the Chennai-Kosasthalaiyar Basin Project
Land Acquisition and Resettlement Due Diligence Report Document Stage: Draft for Consultation Project Number: 49107-009 June 2021 INDIA: Integrated Urban Flood Management for the Chennai-Kosasthalaiyar Basin Project (Infection Prevention and Control of COVID-19 for Integrated Pandemic and Disaster Risk Management for the Urban Poor in the Chennai- Kosasthalaiyar Basin – Under Japan Fund for Poverty Reduction COVID-19 Window) Prepared by Greater Chennai Corporation (GCC) for the Asian Development Bank. CURRENCY EQUIVALENTS (as of 2 June 2021) Currency unit = Indian rupee (₹) ₹1.00 = $0.0137 $1.00 = ₹72.854 ABBREVIATIONS ADB - Asian Development Bank BOD - Biological Oxygen Demand CHS - Chennai Higher School CHSS - Chennai Higher Secondary School CMS - Chennai Middle School COD - Chemical Oxygen Demand CPCB - Central Pollution Control Board CPS - Chennai Primary School DDR - Due Diligence Report DPR - Detail Project Report DMS - Detailed measurement survey GCC - Greater Chennai Corporation GOTN - Government of Tamil Nadu IPC - Infection Prevention and Control JFPR - Japan Fund for Poverty Reduction PPE - Personal Protective Equipment O&M - Operations and Maintenance PMGKY - Pradhan Mantri Gareeb Kalyan Yojana PUPS - Panchayat Union Primary School PUMS - Panchayat Union Middle School SOP - Standard operating procedures UPHC - urban primary health centers WASH - Water Supply Sanitation and Hygiene ZHO - Zonal Head Office WEIGHTS AND MEASURES m – meter mm – millimeter NOTE In this report, "$" refers to United States dollars. This land acquisition and resettlement due diligence report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, management, or staff, and may be preliminary in nature. Your attention is directed to the “terms of use” section on ADB’s website. -

Urban Primary Health Centres (Uphc)
GREATER CHENNAI CORPORATION MEDICAL SERVICES DEPARTMENT URBAN PRIMARY HEALTH CENTRES (UPHC) S.NO ZONE DIVISION LIST OF URBAN PRIMARY HEALTH CENTRES 1 002 Kathivakkam UPHC No.3/392, Kathivakkam High Road, Ennore, Ch – 57 2 004 Ernavoor UPHC BNo.63, All Indio Radio nagar, Tsunami quarters, Ernavoor, Chennai - 57 3 006 Kuppam UPHC No.1,School Street, Jothi Nagar, Ch 19, Thiruvottiyur 4 011 Thiruvottiyur UPHC , No.945, Thiruvottiyur High Road, Ch 19 5 012 Sathangadu UPHC No.9A, Balakrishna Naidu Colony, 2nd Street, Kaladipet, Thiruvottiyur, Ch- 19. 6 014 Thaangal UPHC No.3/2, Poongavanapuram main street, Kaladipet, Chennai - 19 7 015 Manali New Town UPHC,Govt PHC,Manali new town.Ch-103.HP Manali 8 021 Manali UPHC,No.1,Nedunchezhian salai, Manali, Ch-68. 9 022 Puzhal UPHC Gandhi Main Road,Puzhal,Ch-66. HP 10 026 Madhavaram UPHC Swamy Nagar, Madhavaram, Ch-60.HP 11 Madhavaram 031 Kannabiran UPHC, Kannabiran Koil st, Madhavaram, Ch-60. D 12 032 Lakshmipuram UPHC Gangaiamman Koil st, Lakshmipuram, Ch-99.HP 13 34 Kodungaiyur I, No.15, Anna Salai, kodungaiyur, Ch - 118,HP 14 35 Vivekanandha UPHC (Kodungaiyur II), No.39, Appar st, Vivekananda Nagar, kodungaiyur, Chennai – 118 15 036 Sharma Nagar UPHC, No.66,S.A.Colony,8th street,Vyasarpadi, Ch-39, D. 16 037 Vyasarpadi UPHC No. 155A, 14th Cross st, MKB Nagar, Vyasarpadi, ch - 39,HP 17 038 R.K.Nagar UPHC No.140, Annai Sandhya Nagar, 1st street, Tondaiyarpet, Ch-81. 18 039 New washermenpet UPHC, No.4, Cheriyan Nagar 4th st, New Washermenpet, Ch- 81 19 040 V.O.C Nagar (Jeeva Nagar) UPHC, No.1, Chelliamman Koil St, Tondaiyarpet, Ch-81.