Complimentary Contributor Copy Complimentary Contributor Copy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Roberto Cazzolla Gatti – Brief CV
Roberto Cazzolla Gatti – Brief CV Roberto Cazzolla Gatti is Associate Professor in Ecology and Biodiversity at the Biological Institute and Researcher at the Laboratory of Ecology and Biological Diversity of the Tomsk State University, in Russia. He also works as a freelance documentary photographer and wildlife filmmaker. He participates to geographic and scientific explorations of some of the most remote places of Earth. He studied at a Science High School and then graduated in Biology (Bachelor’s Degree), defending a thesis in Marine Ecology (“Demersal Mediterranean marine resources monitoring”), and with highest honours in Environmental and evolutionary biology (Master’s Degree MSc) at the University of Bari, Italy, defending a thesis in Anthropology (“Primate visual system and stereopsis”). He holds a Ph.D. in Forest Ecology earned at the University of Tuscia in Viterbo, studying the tropical forests of Africa and their biodiversity (Ph.D. in Tropical Forest Ecology), defending a thesis titled “The anthropogenic impacts on tropical forest ecology and dynamics”. He also holds with merit a Postgraduate Master’s Degree in International Policies and Global Environmental Protection earned at the Tuscia University in collaboration to the Italian Ministry for the Environment, defending a thesis on “Africa: biodiversity and climate change”. He attended a diploma from the School of Specialization in “Biodiversity and Ecosystem Services” at the Potsdam Institute for Climate Impact Research (PIK), Germany with a training in Peyresq, Alpes de Haute-Provence, France. He is specialized in Biological Diversity analysis and Theoretical-experimental Ecology (with applications in Ethology and Evolution). Moreover, he further developed his studies in Philosophy of Biology, Bioethics and Biochemistry of nutrition. -
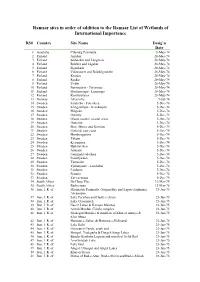
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -
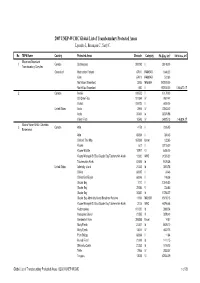
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C. No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Ellesmere/Greenland 1 Canada Quttinirpaaq 300093 II 38148.00 Transboundary Complex Greenland Hochstetter Forland 67910 RAMSAR 1848.20 Kilen 67911 RAMSAR 512.80 North-East Greenland 2065 MAB-BR 972000.00 North-East Greenland 650 II 972000.00 1,008,470.17 2 Canada Ivvavik 100672 II 10170.00 Old Crow Flats 101594 IV 7697.47 Vuntut 100673 II 4400.00 United States Arctic 2904 IV 72843.42 Arctic 35361 Ia 32374.98 Yukon Flats 10543 IV 34925.13 146,824.27 Alaska-Yukon-British Columbia 3 Canada Atlin 4178 II 2326.95 Borderlands Atlin 65094 II 384.45 Chilkoot Trail Nhp 167269 Unset 122.65 Kluane 612 II 22015.00 Kluane Wildlife 18707 VI 6450.00 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 12200 WHC 31595.00 Tatshenshini-Alsek 67406 Ib 9470.26 United States Admiralty Island 21243 Ib 3803.76 Chilkat 68395 II 24.46 Chilkat Bald Eagle 68396 II 198.38 Glacier Bay 1010 II 13045.50 Glacier Bay 22485 V 233.85 Glacier Bay 35382 Ib 10784.27 Glacier Bay-Admiralty Island Biosphere Reserve 11591 MAB-BR 15150.15 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 2018 WHC 66796.48 Kootznoowoo 101220 Ib 3868.24 Malaspina Glacier 21555 III 3878.40 Mendenhall River 306286 Unset 14.57 Misty Fiords 21247 Ib 8675.10 Misty Fjords 13041 IV 4622.75 Point Bridge 68394 II 11.64 Russell Fiord 21249 Ib 1411.15 Stikine-LeConte 21252 Ib 1816.75 Tetlin 2956 IV 2833.07 Tongass 13038 VI 67404.09 Global List of Transboundary Protected Areas ©2007 UNEP-WCMC 1 of 78 No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Tracy Arm-Fords Terror 21254 Ib 2643.43 Wrangell-St Elias 1005 II 33820.14 Wrangell-St Elias 35387 Ib 36740.24 Wrangell-St. -

Geography, M.V
RUSSIAN GEOGRAPHICAL SOCIETY FACULTY OF GEOGRAPHY, M.V. LOMONOSOV MOSCOW STATE UNIVERSITY INSTITUTE OF GEOGRAPHY, RUSSIAN ACADEMY OF SCIENCES No. 01 [v. 04] 2011 GEOGRAPHY ENVIRONMENT SUSTAINABILITY ggi111.inddi111.indd 1 003.08.20113.08.2011 114:38:054:38:05 EDITORIAL BOARD EDITORS-IN-CHIEF: Kasimov Nikolay S. Kotlyakov Vladimir M. Vandermotten Christian M.V. Lomonosov Moscow State Russian Academy of Sciences Université Libre de Bruxelles 01|2011 University, Faculty of Geography Institute of Geography Belgique Russia Russia 2 GES Tikunov Vladimir S. (Secretary-General) Kroonenberg Salomon, M.V. Lomonosov Moscow State University, Delft University of Technology Faculty of Geography, Russia. Department of Applied Earth Sciences, Babaev Agadzhan G. The Netherlands Turkmenistan Academy of Sciences, O’Loughlin John Institute of deserts, Turkmenistan University of Colorado at Boulder, Baklanov Petr Ya. Institute of Behavioral Sciences, USA Russian Academy of Sciences, Malkhazova Svetlana M. Pacific Institute of Geography, Russia M.V. Lomonosov Moscow State University, Baume Otfried, Faculty of Geography, Russia Ludwig Maximilians Universitat Munchen, Mamedov Ramiz Institut fur Geographie, Germany Baku State University, Chalkley Brian Faculty of Geography, Azerbaijan University of Plymouth, UK Mironenko Nikolay S. Dmitriev Vasily V. M.V. Lomonosov Moscow State University, Sankt-Petersburg State University, Faculty of Faculty of Geography, Russia. Geography and Geoecology, Russia Palacio-Prieto Jose Dobrolubov Sergey A. National Autonomous University of Mexico, M.V. Lomonosov Moscow State University, Institute of Geography, Mexico Faculty of Geography, Russia Palagiano Cosimo, D’yakonov Kirill N. Universita degli Studi di Roma “La Sapienza”, M.V. Lomonosov Moscow State University, Instituto di Geografia, Italy Faculty of Geography, Russia Richling Andrzej Gritsay Olga V. -

“Mongolia's Network of Managed Resource
GOVERNMENT OF MINISTRY OF MONGOLIA ENVIRONMENT AND TOURISM “MONGOLIA’S NETWORK OF MANAGED RESOURCE PROTECTED AREAS” МОN/13/303 PROJECT THE PROJECT GOAL: The goal is to ensure integrity of Mongolia’s diverse ecosystems to secure viability of nation’s globally significant biodiversity. IMPLEMENTING PARTNERS: • Ministry of the Environment and Tourism THE PROJECT OBJECTIVE: • United Nations Development Program The project objective is to catalyze strategic expansion of Mongolia’s FUNDING: protected areas system through estab- • Global Environmental Facility – 1,309,091 $ lishment of a network of community • UN Development Program – 200,000 $ conservation areas covering • Ministry of the Environment and Tourism -500,000 $ (in-kind) under-represented terrestrial ecosystems. PROGRAMME PERIOD: TOTAL BUDGET: 2013-2018 1,509,091 $ PROJECT IMPLEMENTATION SITES: 1. “Gulzat” LPA (Uvs aimag, Bukhmurun soums) 203,316 ha 2. “Khavtgar” LPA (Batshireet soum of Khentii aimag) 104,900 ha 3. “Tumenkhan-shalz” LPA (Tsgaaan ovoo, Bayan-uul soums of Dornod aimag, and Norovlin soum of Khentii aimag) 374,499 ha Expanded area under local protection by 600 thousand ha: ACHIEVEMENTS AND OUTPUTS: Expanded area under local protection by about 600.0 thousand hectare and created ecological corridor areas Created under local protection, the ecological corridors/ connectivity support wildlife movement between the state and local pro- 1 tected areas in west and east regions. These are а. 433,9 thousand ha б. 278.9 thousand ha Total of 433,9 thousand ha area, a migration and calv- Argali sheep migration and distribution area of ing land of gazelle located between Tosonkhulstai NR 284.4 thousand ha, that connects Tsagaan shuvuut and Onon Balj NP in Bayan-uul, Tsagaan ovoo and Nor- and Turgen mountain SPAs in Sagil and Buhmurun ovlin soums of Dornod and Khenti aimags; soums of Uvs aimag. -

RCN #33 21/8/03 13:57 Page 1
RCN #33 21/8/03 13:57 Page 1 No. 33 Summer 2003 Special issue: The Transformation of Protected Areas in Russia A Ten-Year Review PROMOTING BIODIVERSITY CONSERVATION IN RUSSIA AND THROUGHOUT NORTHERN EURASIA RCN #33 21/8/03 13:57 Page 2 CONTENTS CONTENTS Voice from the Wild (Letter from the Editors)......................................1 Ten Years of Teaching and Learning in Bolshaya Kokshaga Zapovednik ...............................................................24 BY WAY OF AN INTRODUCTION The Formation of Regional Associations A Brief History of Modern Russian Nature Reserves..........................2 of Protected Areas........................................................................................................27 A Glossary of Russian Protected Areas...........................................................3 The Growth of Regional Nature Protection: A Case Study from the Orlovskaya Oblast ..............................................29 THE PAST TEN YEARS: Making Friends beyond Boundaries.............................................................30 TRENDS AND CASE STUDIES A Spotlight on Kerzhensky Zapovednik...................................................32 Geographic Development ........................................................................................5 Ecotourism in Protected Areas: Problems and Possibilities......34 Legal Developments in Nature Protection.................................................7 A LOOK TO THE FUTURE Financing Zapovedniks ...........................................................................................10 -

Pdf the United Nations, Rome, Italy, Pp
Research Article ii FF o o r r e e s s t t doi: 10.3832/ifor1779-008 Biogeosciences and Forestry vol. 9, pp. 354-362 Does degradation from selective logging and illegal activities differently impact forest resources? A case study in Ghana Gaia Vaglio Laurin (1-3), Degradation, a reduction of the ecosystem’s capacity to supply goods and ser- William D Hawthorne (2), vices, is widespread in tropical forests and mainly caused by human distur- Tommaso Chiti (1-3), bance. To maintain the full range of forest ecosystem services and support the (1) development of effective conservation policies, we must understand the over- Arianna Di Paola , all impact of degradation on different forest resources. This research investi- (1) Roberto Cazzolla Gatti , gates the response to disturbance of forest structure using several indicators: Sergio Marconi (3), soil carbon content, arboreal richness and biodiversity, functional composition Sergio Noce (1), (guild and wood density), and productivity. We drew upon large field and re- Elisa Grieco (1), mote sensing datasets from different forest types in Ghana, characterized by Francesco Pirotti (4), varied protection status, to investigate impacts of selective logging, and of (1-3) illegal land use and resources extraction, which are the main disturbance Riccardo Valentini causes in West Africa. Results indicate that functional composition and the overall number of species are less affected by degradation, while forest struc- ture, soil carbon content and species abundance are seriously impacted, with resources distribution reflecting the protection level of the areas. Remote sensing analysis showed an increase in productivity in the last three decades, with higher resiliency to change in drier forest types, and stronger producti- vity correlation with solar radiation in the short dry season. -

From Sacred Cow to Cash Cow Muller, Martin
From sacred cow to cash cow Muller, Martin License: Creative Commons: Attribution-NoDerivs (CC BY-ND) Document Version Publisher's PDF, also known as Version of record Citation for published version (Harvard): Müller, M 2014, 'From sacred cow to cash cow: the shifting political ecologies of protected areas in Russia', Zeitschrift für Wirtschaftsgeographie, vol. 58, no. 2-3, pp. 127-143. Link to publication on Research at Birmingham portal General rights Unless a licence is specified above, all rights (including copyright and moral rights) in this document are retained by the authors and/or the copyright holders. The express permission of the copyright holder must be obtained for any use of this material other than for purposes permitted by law. •Users may freely distribute the URL that is used to identify this publication. •Users may download and/or print one copy of the publication from the University of Birmingham research portal for the purpose of private study or non-commercial research. •User may use extracts from the document in line with the concept of ‘fair dealing’ under the Copyright, Designs and Patents Act 1988 (?) •Users may not further distribute the material nor use it for the purposes of commercial gain. Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document. When citing, please reference the published version. Take down policy While the University of Birmingham exercises care and attention in making items available there are rare occasions when an item has been uploaded in error or has been deemed to be commercially or otherwise sensitive. -

Îõðàíÿåìûå Ïðèðîäíûå Òåððèòîðèè(Àíãë).Pm6
PROTECTED AREAS IN RUSSIA: LEGAL REGULATION Moscow v 2003 2 Protected Areas in Russia: Legal Regulation. An Overview of ederal Laws. Edited by A.S.Shestakov. KMK Scientific Press Ltd., Moscow, 2003. xvi + 352 p. Reviewer: Dr. O.A.Samonchik, Devision of Agrarian and Land Laws, Institute of State and Law, Russian Academy of Sciences G.A. Kozulko, international expert on protected areas, Chief of the Belovezhskaya Puzha XXI Century Public Initiative This research and publication have been made possible by funding from The Service for Implementation of National Biodiversity Strategies and Action Plans (Biodiversity Service) established by a consortium of United Nations Environment Programme (UNEP), the World Conservation Union (IUCN), the European Centre for Nature Conservation (ECNC) and the Regional Environmental Centre for Central and Eastern Europe (REC) ã 2003 WW Russia ã 2003 M.L.Kreindlin, A.V.Kuznetcova, V.B.Stepanitskiy, A.S.Shestakov, ISBN 5-87317-132-7 E.V.Vyshegorodskih 3 LIST O ACRONIMS ATNM area of traditional nature management of indigenous peoples of the North, Siberia and the ar East of the Russian ederation IUCN The World Conservation Union MNR Ministry of Natural Resources of the Russian ederation NP national park PA protected area R Russian ederation SPNA specially protected natural area SSNR state strict nature reserve (zapovednik) WW World Wide und for Nature 4 TABLES AND IGURES Tables: Table 1. Number and Area of Specially Protected Natural Areas in Russia (as of the beginning of 2003) Table 2. Matrix of Management Purposes of Specially Protected Natural Areas of Russia Table 3. Proposals for Establishing State Strict Nature Reserves and National Parks in the Russian ederation for 2001-2010 Table 4. -

Features of the Demographic Structure and the Condition of Populations of the Rare Relic Hedysarum Gmelinii Ledeb
PROCEEDINGS OF THE LATVIAN ACADEMY OF SCIENCES. Section B, Vol. 74 (2020), No. 6 (729), pp. 385–395. DOI: 10.2478/prolas-2020-0051 FEATURES OF THE DEMOGRAPHIC STRUCTURE AND THE CONDITION OF POPULATIONS OF THE RARE RELIC HEDYSARUM GMELINII LEDEB. (FABACEAE) IN PERIPHERAL AND CENTRAL PARTS OF ITS DISTRIBUTION RANGE Larisa M. Abramova1,#, Valentina N. Ilyina2, Anna E. Mitroshenkova2, Alfia N. Mustafina1, and Zinnur H. Shigapov1 1 South-Ural Botanical Garden-Institute UFIC RAS, 195/3 Mendeleev Str., Ufa, 450080, RUSSIA 2 Samara State University of Social Sciences and Education, 26 Antonova-Ovseenko Str., Samara, 443090, RUSSIA # Corresponding author, [email protected] Communicated by Isaak Rashal The features of the ontogenetic structure of cenopopulations of a rare species Hedysarum gmelinii Ledeb. (Fabaceae) were studied on the periphery of its range (the Middle Volga region and the Bashkir Cis-Urals) and in its central part (the Altai Mountains region). Types of cenopopulations were determined according to the “delta-omega” criterion: in the Bashkir Urals, they were mostly young, in the Middle Volga region, they were mature, in the Altai Mountains, they were maturing. The proportion of pregenerative individuals in populations increases in habi- tats with high moisture levels. Anthropogenic load (mainly in the form of grazing) had a greater ef- fect on the number and density of individuals, rather than on the type of ontogenetic spectrum of cenopopulations. Key words: Hedysarum gmelinii Ledeb., cenopopulation, ontogenetic spectrum, demographic -

The Big Plan
World CONSERVATION THE MAGAZINE OF THE INTERNATIONAL UNION FOR CONSERVATION OF NATURE January 2011 The Big Plan Ocean futures Curbing wildlife trade Love not loss WORLD CONSERVATION Volume 41, No. 1 January 2011 Rue Mauverney 28 1196 Gland, Switzerland Contents Tel +41 22 999 0000 Fax +41 22 999 0002 [email protected] Your space 3 www.iucn.org/worldconservation The turning tide 4 Editor: Anna Knee Managing Editor: John Kidd Production and distribution: Cindy Craker NEW CHALLENGES Contributing editors: A new idealism 5 Deborah Murith Stephanie Achard We need to unplug from virtual reality and reconnect with nature if we have a chance to save biodiversity, says Jeffrey A. McNeely Design: L’IV Comm Sàrl, Le Mont-sur-Lausanne, Getting tough on trade 7 Switzerland Richard Thomas describes the armoury of tools needed to tackle escalating levels of wildlife Printed by: Sro-Kundig, Geneva, Switzerland trade Opinions Staying power 8 Opinions expressed in this publication do not David Huberman examines the rapid rise of the Green Economy concept necessarily refl ect the views of IUCN, its Council or its Members. Comments and suggestions NEW APPROACHES Please e-mail the World Conservation team at [email protected], or telephone us on +41 22 999 0116. There’s no going back 10 Sue Mainka on why conservationists may need to rethink their priorities Back issues An easy win 12 Back issues of World Conservation are available at: www.iucn.org/worldconservation Don’t ignore the cost-effective solution that protected areas offer in tackling climate change and saving biodiversity, says Ernesto Enkerlin-Hoefl ich Paper Feel the love 14 This magazine is printed on FSC paper. -

Invasive and Potentially Invasive Plant Species in State Nature Biosphere Reserves of the Altai Republic (Russia)
Acta Biologica Sibirica, 2019, 5(4), 73-82, doi: https://doi.org/10.14258/abs.v5.i4.7059 RESEARCH ARTICLE Invasive and potentially invasive plant species in State Nature Biosphere Reserves of the Altai Republic (Russia) I. A. Artemov¹,²*, E. Yu. Zykova¹ ¹Central Siberian Botanical Garden, Siberian Branch of the Russian Academy of Sciences Novosibirsk, Russia ²Katunskiy State Nature Biosphere Reserve Ust’-Koksa, Altai Republic, Russia E-mail: [email protected], [email protected] In the Altaiskiy and Katunskiy State Nature Biosphere Reserves we registered 44 alien plant species, which were considered in Siberia as invasive and potentially invasive. Among them, there were 30 xenophytes and 14 ergasiophytes species. Rumex acetosella L., Impatiens glandulifera Royle, Galinsoga ciliata (Rafin.) Blake, and Strophiostoma sparsiflorum (Mikan ex Pohl) Turcz. are considered invasive in the Altaiskiy Reserve because they actively spread there in natural and seminatural plant communities and habitats. Most of the species had appeared in the territories of the reserves before their establishment as a result of agricultural activity or appeared after their establishment because of activity of the reserves themselves. Despite of a big amount of tourists in the reserves, the invasive and potentially invasive plants are absent on the ecological paths at present. Keywords: alien plant species; invasive plant species; Altaiskiy Reserve; Katunskiy Reserve; recreation Introduction Invasion of alien species is the global problem, which is strengthening in XXI century (Tittensor et al. 2014; Early et al. 2016). By now 14000 alien plant species are known, 13000 of which have already naturalized in at least one region of the world (van Kleunen et al., 2015, 2019).