Non-Reciprocal Intragenic Mitotic Recombination in Drosophila
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
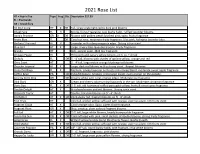
2021 Rose List HT = Hybrid Tea Type Frag Dis
2021 Rose List HT = Hybrid Tea Type Frag Dis. Description $27.99 FL = Floribunda GR = Grandiflora All My Loving HT X DR Tall, large single light red to dark pink blooms Angel Face FL X Strong, Citrus Fragrance. Low bushy habit, ruffled lavander blooms. Anna's Promise GR X DR Blooms with golden petals blushed pink; spicy, fruity fragrance Arctic Blue FL DR Good cut rose, moderate fruity fragrance. Lilac pink, fading to lavander blue. Barbara Streisand HT X Lavender with a deep magenta edge. Strong citrus scent. Blue Girl HT X Large, silvery liliac-lavender blooms. Fruity fragrance. Brandy HT Rich, apricot color. Mild tea fragrance. Chicago Peace HT Phlox pink and canary yellow blooms on 6' to 7' shrub Chihuly FL DR 3' - 4' tall, blooms with shades of apricot yellow, orange and red Chris Evert HT 3' - 4' tall, large melon orange blushing red blooms Chrysler Imperial HT X Large, dark red blooms with a strong scent. Repeat bloomer Cinco De Mayo FL X Medium, smoky lavender and rusty red-orange blend, moderate sweet apple fragrance Coffee Bean PA DR Patio/Miniature. Smokey, red-orange inside, rusty orange on the outside. Coretta Scott King GR DR Creamy white with coral, orange edges. Moderate tea fragrance. Dick Clark GR X Cream and cherry color turning burgundy in the sun. Moderate cinnamon fragrance. Doris Day FL X DR 3'-5' tall, old-fashioned ruffled pure gold yellow, fruity & sweet spice fragrance Double Delight HT X Bi-colored cream and red blooms. Strong spice scent. Elizabeth Taylor HT Double, hot-pink blooms on 5' - 6' shrub Firefighter HT X DR Deep dusky red, fragrant blooms on 5' - 6' shrub First Prize HT Very tall, golden yellow suffused with orange, vigorous plant, rich fruity scent Fragrant Cloud HT X Coral-orange color. -

By JACK SCHULTZ
VOL. 18, 1932 GENETICS: J. SCHULTZ 485 THE BEHA VIOR OF VERMILION-SUPPRESSOR IN MOSAICS By JACK SCHULTZ CARNEGIE INSTITUTION OF WASHINGTON, RESIDENT AT THE CALIFORNIA INSTITUTE OF TECHNOLOGY, PASADENA, CALIFORNIA Communicated June 13, 1932 It is a point of interest whether the suppressors of the effects of mutant genes affect development in the same way as do the wild-type allelomorphs of these genes, or behave in some other manner. The case of vermilion- suppressor in Drosophila melanogaster presents the advantage for the study of this problem that the suppressor is known to be a recessive mutant gene at another locus, and not a duplicating wild-type allelomorph of vermilion (Schultz and Bridges, 1932). The gene vermilion has the peculiarity (Sturtevant, 1920) that, in mosaics, not only the genetic constitution of the eye, but also that of the rest of the fly, determines the color of the mosaic patch. Tissue that is genetically wild type in such a mosaic, however, is "self-differentiating;" its characteristics are determined, as Sturtevant points out, by its own genetic constitution. The behavior of vermilion-suppressor in mosaics therefore provides a test of the similarity of its behavior to that of the wild-type allelomorph of vermilion. Two series of experiments were carried out. In one set, males con- taining the genes vermilion-suppressor, apricot, crossveinless, vermilion and forked, were made heterozygous for a duplication covering the sup- pressor. This duplication (134), found in an x-ray experiment by Dr. Th. Dobzhansky, belongs to the group of eversporting chromosome re- arrangements, and thus frequently gives mosaic patches. -

Apricot (MEKO03) Nut (MEKO04)
(41F) Koru is an interpretation of the wood found in fruit trees, characterized by light veining and distinctive knots. The name Koru derives from a Maori word and symbol representing new life, growth and peace. The colors of Mirage’s Koru are clean and crisp, creating a final look that creates a peaceful living space. The classic, natural colors are further enhanced by the opaque surface that absorbs light rather than reflecting it. The innovative finish and the high number of different images make this wood look porcelain incredibly realistic and universally appealing. Apple (MEKO01) Peach (MEKO02) Apricot (MEKO03) Nut (MEKO04) Colors are intended as a guide only and may vary from actual tile. Sizes listed are nominal. Please check samples before making final selection and to get actual dimensions for layout. See reverse side for additional product information. PRODUCT INFORMATION Size - Rectified 8 x 48 MEKO--/848 Colors MEKO01 Apple (White/Ivory) MEKO02 Peach (Greige) MEKO03 Apricot (Beige/Honey) MEKO04 Nut (Walnut) INSTALLATION INFORMATION We recommend using a tile leveling system to install this product Before installing the tile you should: • Ensure the perfect planarity (flatness) of underlying floor base. • Always double-check the quality of the tiles. • When setting, tile should be only slightly staggered so that the ends of the tiles are within 8” or less from the ends of the corresponding staggered tile. Figure 1 (See figures 1 & 2) • Staggered spacing does not need to be uniform, but should not exceed 8” recommendation. • It is preferable to use a grouting material that matches the color of the tiles. -

Annual Plant List 2021
Annual Plant List 2021 VARIETY COLOR HEIGHT LIGHT Pack/Pot Price Tray Price AGERATUM Aguilera Violet violet 10-12" sun/pt sun $3.99 Aloha Blue blue 6" sun/pt shade $2.59 $18.99 ALLAMANDA Brown Bud yellow vining sun $34.99 ALLYSUM Snow Crystals white 3" sun/pt shade $2.59 $18.99 Wonderland Deep Purple purple 2-4" sun/pt shade $2.59 $18.99 Wonderland Mix mix 2-4" sun/pt shade $2.59 $18.99 ALSTROEMERIA Inca Lolly hot pink 10-12" sun $9.99 Inca Miss Zoe orange-red 10-12" sun $9.99 Inca Sundance yellow 12" sun $9.99 ANGELONIA Angelface Blue PW blue 18-24" sun $3.99 Angelface Perfectly Pink PW pink 18-30" sun $3.99 Angelface Steel Blue PW lavender 18-30" sun $3.99 Angelface Wedgewood Blue PW blue 18-30" sun $3.99 Angelface White PW white 18-24" sun $3.99 ARGYRANTHEMUM Golden Butterfly PW yellow 18-36" sun $7.99 Pure White Butterfly PW white 18-36" pt sun/sun $7.99 Sassy Rose pink 18-24" sun $7.99 ASCLEPIAS currasavica tricolor 36" sun $4.99 ASPARAGUS FERN Sprengeri green trailing sun $3.99 BEGONIA Double Delight Blush Rose PW salmon pink 8-14" sun/shade $4.99 Pegasus PW green 12-18" pt shade/shade $4.99 BEGONIA, boliviensis Beauvilia White white trailing sun/part shade $4.99 Mistral Pink pink trailing sun/pt shade $4.99 Mistral Red red trailing sun/pt shade $4.99 Mistral Yellow yellow trailing sun/pt shade $4.99 Waterfall Orange orange trailing sun/pt shade $4.99 BEGONIA, Fibrous Nightlife Mix mix/brz leaves 8-10" sun/pt shade $2.59 $18.99 Nightlife Pink pink/brz leaves 8-10" sun/pt shade $2.59 $18.99 Nightlife Red red/brz leaves 8-10" sun/pt -

Fairview South Plant Sale
Fairview South Plant Sale Spring is in the air and Fairview South will be selling flowers and vegetables for our spring fundraiser. The sale will run from Friday, March Ist through Friday, March 22nd. Proceeds from the sale will be used to fund the 2019 Graduation. All flowers and vegetables are grown locally by the students at Genesee Lake School, Homegrown Farms, as part of their vocational training. * Please fill out the attached form(s) and return the form(s) along with payment to Fairview South no later than Friday, March 22nd. We will not be able to accept money the day of pickup. + Payment should be turned in with your order forms). Cash or checks made out to Fairview South, are accepted. Don't forget to please add $2.00 per order for the delivery fee. Flowers and vegetables will be delivered to Fairview South and will be available for pickup in the Fairview South Gym on Wednesday, May 8th between 1:00 and 6:00. Questions? Please contact Angela Sidebottom at [email protected] or Beth Freydank at [email protected]. We can also be reached at Fairview South at (262) 781-9464. Thank you for your support and happy planting! Angela and Beth HOMEGROWN FARMS - SPRING 2019 GREENHOUSE PHONE (262) 569-5531 • CELL (414) 581-4532 • FAX (262) 569-5513FVS [\]3ff)€ _(gfl _ PfOp)€ > GERANIUMS - 4.5" FLATS (48 Plants) GRASSES - 6.5" Price $.4,7F Price s /Io.DD Price 54. 76 Pink Alyssum-White Purple Fountain Grass Purple Begonia (Bronze)-Red Corkscrew Big Twister Red Begonia (Bronze)-Rose [,, Total # of plants Salmon/coral Begonia -

Peach, Apricot, and Nectarine
Peach, Apricot, and Nectarine plantdiseasehandbook.tamu.edu/food-crops/fruit-crops/peach-apricot-and-nectarine/ Armillaria Root Rot: This can be a major disease in older orchards and replanted orchards. (See Photo) (See Section on Mushroom Root Rot) Bacterial Canker (bacterium – Pseudomonas syringae): Elongated cankers develop at the base of buds and randomly on the trunk and scaffold limbs (See Photo). Damaged areas are slightly sunken and somewhat darker in color than the surrounding bark. At both the upper and lower margins of the canker, narrow brown streaks extend into healthy tissue. As the trees break dormancy in the spring, gum is formed by the surrounding tissue and may exert enough pressure to break through the bark and flow. The area beneath the canker has a soured odor. Individual scaffolds or the entire tree usually dies shortly after leafing out in the spring. Roots are not affected. Extensive suckering (See Photo) often occurs at the tree base. The bacterium is a weak pathogen and causes serious damage only when a tree is in a dormant condition or weakened due to unfavorable growing conditions. Bacterial canker is a component in a disease complex known as Peach Tree Short Life. Trees up to 7 years old, growing on deep sandy soil are most susceptible. Avoid using high nitrogen fertilizer rates in mid to late summer. Do not encourage late fall growth. Prune when the trees are fully dormant (January and February). High dosage of a copper-containing fungicide at leaf drop has been somewhat successful. Bacterial Spot (bacterium – Xanthomonas campestris pv. pruni): Symptoms on leaves are observed first as small, circular, or irregularly shaped, pale green lesions (See Photo). -

Liquicolor Permanente Shades 07-10-12.Pdf
LIQUICOLOR PERMANENTE INTENSE INTENSE INTENSE INTENSE GOLD RED NEUTRAL (RN) INTENSE RED (RR) RED VIOLET (RV) RED VIOLET (RRV) BASE INTENSE NEUTRAL (NN) NEUTRAL (N) ASH (A) ASH (A) ASH (AA) ASH (AA) NEUTRAL(GN) GOLD (G) REDPELIRROJO NEUTRAL (RN) RED (R) INTENSEPELIRROJO RED (RR) REDPELIRROJO VIOLET (RV) REDPELIRROJO VIOLET (RRV) NEUTRAL INTENSO NEUTRAL CENIZO CENIZO CENIZO INTENSO CENIZO INTENSO DORADO NEUTRAL GOLDDORADO (G) PELIRROJONEUTRAL PELIRROJORED (R) PELIRROJOINTENSO PELIRROJOVIOLETA VIOLETAPELIRROJO INTENSO LEVEL/NIVEL DORADO NEUTRAL PELIRROJO INTENSO VIOLETA VIOLETA INTENSO 12 HIGH-LIFT BLONDE 12N/HL-N 12A/HL-V 12AA-B/HL-B 12AA-BV 12G / HL-G HIGH LIFT HIGH LIFT HIGH LIFT ULTRA HIGH LIFT ULTRA HIGH LIFT GOLDEN BLONDE NEUTRAL BLONDE COOL BLONDE COOL BLONDE BLUE COOL BLONDE BLUE VIOLET 10 LIGHTEST BLONDE RUBIO CLARÍSIMO 10N/12B1 10GN/12G2 OUR FIRST NN 10G / 12G LIGHTEST NEUTRAL BLONDE LIGHTEST GOLD LIQUID COLOR LIGHTEST GOLDEN BLONDE blondest beige NEUTRAL BLONDE blondest blonde blondest gold 9 VERY LIGHT BLONDE RUBIO MUY CLARO 9NN 9N/89N 9A/26D 9A-N/40D 9AA/20D 9AA-BV/30D VERY LIGHT RICH VERY LIGHT VERY LIGHT VERY LIGHT COOL VERY LIGHT ULTRA VERY LIGHT ULTRA COOL NEUTRAL BLONDE NEUTRAL BLONDE COOL BLONDE NEUTRAL BLONDE COOL BLONDE BLONDE BLUE VIOLET lightest neutral blonde winter wheat topaz arctic blonde flaxen blonde 8 LIGHT BLONDE RUBIO CLARO 8NN 8N/88N 8A / 28D 8GN/27G 8RN / 71RG 8R / 29R LIGHT RICH LIGHT LIGHT COOL BLONDE LIGHT GOLD NEUTRAL LIGHT RED NEUTRAL LIGHT RED BLONDE NEUTRAL BLONDE NEUTRAL BLONDE autumn mist BLONDE -

Assessment of Apricot Color and Quality Changes Using Color Indices
Volume 18(4), 70- 73, 2014 JOURNAL of Horticulture, Forestry and Biotechnology www.journal-hfb.usab-tm.ro Assessment of apricot color and quality changes using color indices Petrisor Cristina Research and Development Station for Fruit Tree Growing Baneasa, Bdv. Ion Ionescu de la Brad, No.4, Sect.1, Bucharest, Romania *Corresponding author. Email: [email protected] Abstract In apricot fruit, establishing the optimal harvest time is crucial, Key words since fruit quality potential are closely related to the ripening stage at harvest. Stage of maturation is usually estimated by fruit color through parameter apricot, chromatic L*,a*, b*. The goal of this study was establish feasibility of different chromatic parameters, carotenoids, indices for apricot color and quality determination and their relationship with color index pigments and ethylene concentration. Color changes during apricot ripening were the result of significant changes in the values of a*, h°, ( a*/b*)2, (1000a*/L*b*), (180- h°/L*+C*). Chroma and lightness were not a good parameters to express apricot ripeness because narrow range of variation and small differences between stages of maturation and even between varieties. Relationship between b*, L*,C* and total carotenoids content were very weak. For ripened apricot fruits a*, h° and chromatic indices could be used as objective ripening indices. Color is considered to be one of the most important classify the red grapes depending on their external external factors of fruit quality, as the appearance of color. This index showed a good linearity with the the fruit greatly influences consumers [10]. visual colour of the fruits and distinguished between The study of the variability of the apricot sample groups of different external color. -

Scab of Peach, Nectarine, Plum, and Apricot
report on RPD No. 811 PLANT September 1988 DEPARTMENT OF CROP SCIENCES DISEASE UNIVERSITY OF ILLINOIS AT URBANA-CHAMPAIGN SCAB OF PEACH, NECTARINE, PLUM, AND APRICOT Scab, also known as black spot or freckles, is caused by the fungus Cladosporium carpophilum. The disease occurs throughout the Midwest, wherever peaches, nectarines, plums, and apricots are grown. Nectarines, plums, and apricots are less affected than peaches. The principal loss from scab is the spotting of the fruit skin. Minor losses occur from premature defoliation and a dieback of the twigs. Warm, wet conditions during the spring and early summer after petal-fall are necessary for a severe infection by the scab fungus. Figure 1. Typical dark lesion of scab with characteristic Scab is most common in home orchards where no halo on mature peach fruit (courtesy D.F. Ritchie). specific fungicide control program is practiced. The general use of fungicides by commercial growers has reduced the loss from scab; it is a minor disease, except in very wet seasons on poorly pruned trees with poor orchard sanitation. The disease is usually more serious in low-lying, moist, shady areas where evaporation from fruit surfaces is poor. SYMPTOMS Fruit. Scab first appears on the fruit about 6 or 7 weeks after the petals have fallen, when the fruit are half-formed to nearly full grown. Small, round, olive green spots about 1/16 to 1/8 inch in diameter develop on the surface of the fruit (Figure 1). Scab is most common near the stem end or on the side of the fruit exposed to the sun. -

APRICOT and ALMOND BROWN ROT 887 Two Brown Rot Diseases
886 YEARBOOK OF AGRICULTURE 1953 and with a nearly airtight pack or container. For prepackaged flowers or for flowers stored in water in a moder- ately tight cold room, the potential damage that can be done if ethylene- Apricot and producing diseased material is in- cluded is serious. Observations have indicated that once a blossom is in- Almond jured by ethylene it immediately becomes more liable to attack by Botrytis. Thus a chain-reaction type Brown Rot of response is initiated that will lead to more ethylene production and thus to more injury. E. E. Wilson The observed effects of ethylene produced by diseased plant tissues Probably nowhere else in the world emphasize the desirability of either are stone fruits grown in such variety near-perfect control or complete elim- and number as in the three States that ination of plant disease. If adequate border the Pacific Ocean. There are disease control is maintained in field grown the edible varieties of peaches, or greenhouse and in the storage nectarines, apricots, the three types of room, one of the important factors in cherries, plums, prunes, and almonds. successful long-term storage of cut There, too, are produced the numerous flowers is reduced to negligible pro- other species of stone fruits that are uti- portions. lized as rootstocks for the edible sorts. The orchards in the region are com- G. E. WILLIAMSON, assistant professor posed not of a few trees of miscellaneous of plant pathology in Cornell University^ is kinds grown in the back yards of scat- a native of Indiana. His work with dis- tered farm homes but many trees of the eases of ornamental plants began at Cornell same kind in contiguous blocks ex- in igsy. -

Backyard Tree Catalog
201 9 Backyard Tree Catalog Fowler Nurseries has been producing fruit trees in Placer County since 1912. We are a leader in our industry, which serves production agriculture. In order to stay on top, we travel the world discovering new and exciting Varieties and Rootstocks. Fowler Nurseries is pleased to make available to the homeowner the best of our discoveries as well as the best of the “old tried and true” HEIRLOOM selections. We hope your shopping experience is enjoyable and informative. Featuring: • Almonds OPEN! • Apricots • Asian Pears January 11th through • Cherries March 2nd 2019 • Nectarines FRIDAYS AND • Plums - Fresh and Dried • Peaches - Freestone SATURDAYS ONLY • Pears 8:00am – 4:00pm How We Ensure You Always Receive A Superior Tree • Careful selection of seed or rootstock • Strict attention to trueness of variety • Grown in mineral-rich, decomposed granite soil, which creates a fibrous root system • Superior scion wood or bud wood • Exact staking of trees to ensure straight trunks • Use a specialized nutrient formula to develop trees • Precise trimming of suckers and branches to promote good healing • Strict high-quality standards at harvest and shipping 525 FOWLER ROAD NEWCASTLE, CA 95658 (916) 645-8191 www.fowlernurseries.com ALMONDS Apricots, continued. Nicole Teeming with essential minerals and healthy nutrients, Standard and Semi-Dwarf Early June almonds are enjoyed in a wide variety of commercial products. ~Really Sweet!~ Most almonds are grown in California because of its Very sweet, highly flavored apricot with low acidity. Smaller Mediterranean-type climate. fruit than other apricots but, packed with flavor. Not self- fertile. Use Apache, Robada or Royal as pollinizers. -

New Varieties 2022
New Varieties 2022 Nonstop Joy® Red People. If you want the best flower seeds in the industry for your business, you‘ve come to the right place! Plants. With an almost 200 year old legacy as a family-owned business, Innovation. you might think of Benary as an old or old-fashioned company... On the contrary. Throughout our long history, world events have played a significant role in the necessity to continuously reinvent ourselves, giving us the spirit of a multigenerational startup. Despite many challenges and transitions throughout the years, Benary has not only survived, every obstacle we have faced has made us stronger, better. Together we are striving to be the most professional, innovative and personal company in global floriculture. Thank you for working with us! Your Benary Team Contact us– we are committed to your success! Matthias Mart Jennifer Calhoun Doreen Rowe Heidi Sweeney Sales & Marketing Manager NA Marketing Specialist Operations Manager DeKalb Customer Service Assistant / [email protected] [email protected] [email protected] Inventory Planner [email protected] Please contact us for further assistance. The North American Benary team is available to support you and assist with your individual needs. We are committed to your success and are happy to answer any questions. Our seed storage and distribution departments in DeKalb, IL, USA provide fast order processing and prompt shipping to our customers. Nonstop Joy® Rose Picotee Table of Contents ® TM ® Ptilotus Ptilotus exaltatus Joey Tagetes patula Super Hero Viola wittrockiana F1 Inspire DeluXXe 15 16 20 Increase your Sales with Joey® The next Generation of Heroes! Indulge your Customers in Luxury More compact and branching plant habit – Super HeroTM! Perfect for late summer and early full of flower spikes.