Acute and Chronic Whiplash Disorders – a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Posterior Dislocation of Hip in Adolescents Attributable to Casual Rugby
J Accid Emerg Med 2000;17:429–431 429 J Accid Emerg Med: first published as 10.1136/emj.17.6.430 on 1 November 2000. Downloaded from EMERGENCY CASEBOOKS Posterior dislocation of hip in adolescents attributable to casual rugby K Mohanty, S K Gupta, A Langston A 11 year old boy was brought to the accident eight weeks and magnetic resonance imaging and emergency department with a painful left of the hip at six months ruled out avascular hip after having been injured it in a tackle in a necrosis of the head of femur. casual game of rugby. On examination the hip Posterior dislocation of hip usually occurs was found to be flexed, adducted and inter- when force is directed proximally up the shaft nally rotated with no distal neurovascular defi- of femur from knee to the flexed hip1. Although cit. All movements of that hip were extremely it is commonly seen after high energy road Department Of painful. Posterior dislocation of hip was traYc accidents, it can occur in children result- Trauma and confirmed by radiograph (fig 1).This was ing from relatively minor injury such as a Orthopaedics, reduced under general anaesthesia within three Morriston Hospital, casual game of rugby as reported here. Such Swansea hours of the injury. After reduction he was on dislocations have been reported attributable to skin traction for a week and followed by jogging, skiing, mini rugby2 and basketball. Correspondence to: non-weight bearing mobilisation for a further Major complications of traumatic hip disloca- Mr Mohanty, 65 Hospital four weeks. Computed tomography was done tion include nerve injury, avascular necrosis of Close, Evington, Leicester LE54 WQ (Kmohanty@ to rule out any intra-articular bone fragments. -

Physio Med Self Help for Achilles Tendinopathy
Physio Med Self Help 0113 229 1300 for Achilles Tendinopathy Achilles tendon injuries are common, often evident in middle aged runners to non-sporting individuals. They are often characterised by pain in the tendon, usually at the beginning and end of exercise, pain and stiffness first thing in the morning or after sitting for long periods. There is much that can be done to both speed up the healing and prevent re-occurrence. Anatomy of the Area The muscles of your calf (the gastrocnemius and soleus) are the muscles which create the force needed to push your foot off the floor when walking, running and jumping, or stand up on your toes. The Achilles tendon is the fibrous band that connects these muscles to your heel. You may recognise the term ‘Achilles Tendonitis’ which was the previous name used for Achilles Tendinopathy. However the name has changed as it is no longer thought to be a totally inflammatory condition, but rather an overuse injury causing pain, some localised inflammation and degeneration of the thick Achilles tendon at the back of the ankle. Potential causes of Achilles Tendinopathy and advice on how to prevent it • Poor footwear or sudden change in training surface e.g. sand makes the calf work harder » Wear suitable shoes for the activity (type, fit and condition of footwear). » Take account of the surface you are exercising on and if soft and unstructured like sand or loose soil reduce the intensity / duration or take a short break or reduce any load you are carrying into smaller loads until you become conditioned to it. -

An Evidence Based Medicine Understanding of Meniscus Injuries and How to Treat Them
An Evidence Based Medicine Understanding of Meniscus Injuries and How to Treat Them Patrick S. Buckley, MD University Orthopaedic Associates June 1, 2019 Disclosures • None www.UOANJ.com Anatomy of the Meniscus • Act as functional extensions of the tibial plateaus to increase depth of tibial articular surface • The meniscotibial attachment contributes to knee stability • Triangular in cross-section Gross Anatomy of the Meniscus • Ultrastructural Anatomy – Primarily Type I collagen (90%) – 70% water – Fiber orientation is circumferential (hoop stressing) Meniscal Vascularity • Relatively avascular • Vascular penetration – 10 - 30% medial – 10 - 25% lateral • Non-vascularized portions gain nutrients from mechanical loading and joint motion Medial Meniscus • Semilunar shape • Thin anterior horn • Broader posterior horn • More stable & less motion than the lateral = tears more often Lateral Meniscus • Almost circular in shape • Intimately associated with the ACL tibial insertion • Posterior horn attachments – Ligament of Humphrey – Ligament of Wrisberg • Lateral meniscus is a more dynamic structure with more motion Main Importance of Menisci • Load transmission • Joint stability Load Bearing / Shock Absorption • MM 50% and 70% LM of load transmitted through mensicus in extension • 85 % at 90° of flexion • Meniscectomy – 50 % decrease in contact area – 20 % less shock absorption www.UOANJ.com Meniscal effect on joint stability • Secondary restraints to anterior tibial translation in normal knees • In an ACL-deficient knee: the posterior horn of the medial meniscus is more important than the lateral meniscus Interaction of ACL and PHMM • Lack of MM in ACLD knees significantly ↑ anterior tibial translation at all knee flexion angles • Think about with high grade pivot! (Levy, JBJS,1982) (Allen, JOR,2000) C9ristiani, AJSM, 2017) Meniscus Function -Now known to be important structure for load distribution and secondary stabilizer to the knee. -

Rehabilitation Advice Following a Whiplash Injury
Further Information If you require any further information after reading this leaflet, please contact: Therapies Department Tel: 01926 608068 As a key provider of healthcare and as an employer, the Trust has a statutory obligation to promote and respect THERAPIES SERVICE equality and human rights. This is set out in various pieces of legislation including: Race Relations (Amendment) Act 2000, Disability Discrimination Act (2005), Sex Discrimination Act (1975) and the Age Discrimination Act Rehabilitation Advice (2006) Our information for patients can also be made available in following a Whiplash other languages, Braille, audio tape, disc or in large print. Injury PALS We offer a Patient Advice Liaison Service (PALS). This is a confidential service for families to help with any questions or concerns about local health services. You can contact the service by the direct telephone line on 01926 600 054 or calling in at the office located at Warwick Hospital. Date: January 2016 Revision Due: January 2019 Author: Outpatient Physiotherapy Team Leader SWH 01390 If you are unable to attend your appointment please telephone 01926 608068 to cancel your appointment Introduction Neck movement exercises: Sit in the correct postural position, as in exercise 3 repeat all What is whiplash? exercises below 10 times to each side. ‘Whiplash’ is the term used to describe when your head moves quickly forward and then backwards, which commonly 5. Rotation happens in road traffic accidents. This quick back and forth Gently turn your head from one side to the other. Your eyes movement may cause injury to the neck should follow the direction in which you are turning. -

Platelet-Rich Plasma Prolotherapy for Low Back Pain Caused By
Prolotherapy Platelet-Rich Plasma Prolotherapy for Low Back Pain Caused by Sacroiliac Joint Laxity A relatively new treatment modality, PRP prolotherapy demonstrates effectiveness in case studies of patients with sacroiliac (SI) joint ligament laxity and painful dysfunction. Donna Alderman, DO Platelet-rich plasma prolotherapy (PRPP) is an injection treatment that stimulates healing. Like dextrose prolotherapy, PRPP “tricks” the body into repairing incompletely-healed musculoskeletal injuries that results in reduced pain and increased function. Growth factors from blood platelets in platelet-rich plasma stimulate and accelerate healing. Reports are continuing to emerge of the effectiveness, safety, and regenerative capacity of this treatment. In this interesting article, Dr. Gordon Ko, a Canadian physi- cian, shares his expertise in the use of PRPP for low back pain caused by sacroiliac joint laxity. Dr. Ko integrates PRPP with other modalities to accomplish reliable and often dramatic improvement for his patients in this retrospective case report study. — Donna Alderman, DO Prolotherapy Department Head By Gordon D. Ko, MD, CCFP(EM), FRCPC, FABPM&R, FABPM he sacroiliac joints are subject however, quite unreliable.1,2 to con-siderable stresses in A new scale to diagnose SI joint instability that responds to Tweight-bearing and back- prolotherapy has been recently co-developed by the author and twisting movements. Trauma to the SI ligaments can occur with is undergoing validity/reliability testing (Whitmore-Gordons falls on the buttocks, car accidents, twisting and lifting injuries, Sacroiliac Instability Tool; see Appendix A). SI joint dysfunction and repetitive impact loading from excessive running diagnosed by intra-articular blocks accounts for about 20% of (marathoners). -

Ministry of Health of Ukraine Ukrainian Medical Dental Academy
Ministry of Health of Ukraine Ukrainian Medical Dental Academy Methodical instructions for independent work for students during training to practical (seminar) classes and in class Academic discipline Surgical dentistry Module № 5 Lesson topic № 1 Anatomy of the temporomandibular joint (TMJ). Modern methods of diagnosing TMJ diseases. Arthroscopy, its possibilities in the diagnosis and treatment of TMJ diseases. Dislocations of the mandible: etiology, clinic, diagnosis, treatment. Curation of the patient in the clinic of maxillofacial surgery. Writing an academic medical history. Course V Faculty Stomatological Poltava 2020 1. Relevance of the topic. Knowledge of the anatomical structure of the temporomandibular joint (TMJ) and the characteristics of modern diagnostic methods for assessing their pathologies. The etiology, clinical diagnosis and treatment of mandibular dislocations allows you to choose a timely and effective way to treat this pathology, avoid mistakes and complications, allows the dentist to diagnose TMJ and prescribe optimal treatment. Academic history in which the student is able to use knowledge , obtained in the study of basic and applied sciences, obtained demonstrate practical skills. 2. Specific target: 2 .1.Analyze to know statistics, diseases TMJ.; 2.2. Explain the methods of diagnosing diseases TMJ; 2.3. To offer to examine patients with diseases of TMJ; 2.4. Classify diseases TMJ; 2.5. Interpret theoretical and clinical studies of diseases TMJ; 2.6. Draw diagrams, graphs 2.7. Analyze the treatment plan for patients with diseases TMJ; 2.8. Make a plan for the treatment of patients with diseases TMJ; 3. Basic knowledge, skills, abilities necessary for studying the topic (interdisciplinary integration). Names of previous Acquired skills disciplines Anatomy To study the anatomical and topographic structure of the temporomandibular joint. -

ANKLE LIGAMENT STRAIN DURING SUPINATION SPRAIN INJURY – a Alt, W., Lohrer, H., & Gollhofer, A
Vilas-Boas, Machado, Kim, Veloso (eds.) Portuguese Journal of Sport Sciences Biomechanics in Sports 29 11 (Suppl. 2), 2011 REFERENCES: ANKLE LIGAMENT STRAIN DURING SUPINATION SPRAIN INJURY – A Alt, W., Lohrer, H., & Gollhofer, A. (1999). FunctionalProperties of Adhesive Ankle Taping: COMPUTATIONAL BIOMECHANICS STUDY Neuromuscular and Mechanical Effects Before and After Exercise. Foot & Ankle International , 20(4), 238-45. Daniel Tik-Pui Fong1,2, Feng Wei3, Youlian Hong4,5, Tron Krosshaug6, Benesch, S., Putz, W., Rosenbaum, D., & Becker, H.-P. (2000). Reliability of Peroneal Reaction Time Roger C. Haut3 and Kai-Ming Chan1,2 Measurements. Clin Biomech , 15. Cordova, M. L., Bernard, L. W., Au, K. K., Demchak, T. J., Stone, M. B., & Sefton, J. M. (2010). Department of Orthopaedics and Traumatology, Prince of Wales Hospital, Cryotherapy and ankle bracing effects on peroneus longus response during sudden inversion. J 1 Electromyogr Kinesiol , 20, 248-53. Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China The Hong Kong Jockey Club Sports Medicine and Health Sciences Centre, Delahunt, E. (2007). Peroneal reflex contribution to the development of functional instability of the 2 ankle joint. Phys Ther Sport , 8, 98-104. Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China 3 Docherty, C. L., & Arnold, B. L. (2008). Force sence deficits in functionally unstable ankles. J Orthop Orthopaedic Biomechanics Laboratories, Michigan State University, USA Res , 26(11), 1489-93. Department of Sports Science and Physical Education, Faculty of Education, 4 Eechaute, C., Vaes, P., Duquet, W., & Gheluwe, B. v. (2009). Reliability ans discriminative validity of The Chinese University of Hong Kong, Hong Kong, China sudden ankle inversion measurements in patients with chronic ankle instability. -

Whiplash,Vertigo (BPPV),Total Knee Replacement (TKR),Tips for Self-Care of Your Back,Shoulder Impingement,Sever's Disease,Safe
Whiplash What is Whiplash? Whiplash is defined as an acute acceleration/ deceleration injury to the cervical spine (neck), where the head is flung forwards and backwards at high speeds. Whiplash injury can result in damage to the joints within the neck, the bones, the soft tissue surrounding the neck or damage to the neural tissue. It can cause widespread pain to the neck, head, shoulders and arms. How does it happen? Whiplash most commonly occurs in high speed motor vehicle accidents, however it can also occur in sporting activities and falls. What can a physiotherapist do? The physiotherapist will provide a thorough assessment of your neck and thorax, and then determine the extent of your whiplash injury. If a fracture or serious damage is suspected the physiotherapist will refer you for further medical attention and imaging and can refer you for X-rays if required. Initial treatment of a whiplash injury requires rest and avoidance from aggravating activity. Ice and anti- inflammatories may be recommended in the initial phase to reduce swelling. Correct posture is vital to avoid increased strain on the neck and aid recovery. The physiotherapist may provide postural taping or a neck brace to assist with this. The physiotherapist will also provide further treatment to assist in optimal recovery including soft tissue massage, mobilisations, dry needling and electrotherapy. A rehabilitation program will be designed to help increase the movement, strength and stability of your neck and surrounding musculature. The physiotherapist may also provide recommendations on appropriate pillows to provide your neck with the best support whilst sleeping. -

Ankle Sprain Information
DON’T STRAIN YOUR BRAIN WHEN CARING FOR AN ABOUT 28,000 ANKLE INJURIES occur in the United States each day. IT’S BELIEVED • Field hockey has the highest rate of • Ankle sprains are graded on severity ankle injuries and sprains, followed and range from grade 1 (mild; no by volleyball, football, basketball, signifcant structural injury) to grade 45% cheerleading, ice hockey, lacrosse, 3 (severe; complete rupture of the OF ALL ATHLETIC INJURIES soccer, rugby, track and feld, ligamentous structures). ARE ANKLE SPRAINS, making it the most common gymnastics and softball. • After an ankle is sprained, it has a sports injury. • An ankle sprain occurs when there is greater chance of becoming sprained a tear in the ligament, while an ankle again. Repeating ankle sprains strain occurs when there is a tear in put an individual at risk for ankle the muscle. osteoarthritis. KNOWING THE PHASES ACUTE PHASE: Usually the frst two weeks of injury. The ankle will have SUBACUTE PHASE: After the frst two weeks of injury. During this pain, heat, swelling, redness and/or bruising and loss of function. phase, the body begins to heal the damaged tissues of the ankle. By now, the ankle should have regained its range of motion, and should begin to improve in balance and strength. TREATMENT OPTIONS REST ICE COMPRESSION ELEVATION Not all ankle sprains are alike, so be sure to consult a health care provider, such as an athletic trainer or physician, for an individualized treatment plan. HOW TO PREVENT AN ANKLE SPRAIN Have a prevention program created by an athletic Tape or brace ankles during sport activities, such as trainer or qualifed medical provider that focuses games and practices. -

Follow-Up MR Imaging of the Alar and Transverse Ligaments After Whiplash Injury: ORIGINAL RESEARCH a Prospective Controlled Study
Follow-Up MR Imaging of the Alar and Transverse Ligaments after Whiplash Injury: ORIGINAL RESEARCH A Prospective Controlled Study N. Vetti BACKGROUND AND PURPOSE: The cause and clinical relevance of upper neck ligament high signal J. Kråkenes intensity on MR imaging in WAD are controversial. The purpose of this study was to explore changes in the signal intensity of the alar and transverse ligaments during the first year after a whiplash injury. T. Ask K.A. Erdal MATERIALS AND METHODS: Dedicated high-resolution upper neck proton attenuation–weighted MR M.D.N. Torkildsen imaging was performed on 91 patients from an inception WAD1–2 cohort, both in the acute phase and 12 months after whiplash injury, and on 52 controls (noninjured patients with chronic neck pain). Two J. Rørvik blinded radiologists independently graded alar and transverse ligament high signal intensity 0–3, N.E. Gilhus compared initial and follow-up images to assess alterations in grading, and solved any disagreement A. Espeland in consensus. The Fisher exact test was used to compare proportions. RESULTS: Alar and transverse ligament grading was unchanged from the initial to the follow-up images. The only exceptions were 1 alar ligament changing from 0 to 1 and 1 ligament from 1 to 0. The prevalence of grades 2–3 high signal intensity in WAD was thus identical in the acute phase and after 12 months, and it did not differ from the prevalence in noninjured neck pain controls (alar ligaments 33.0% versus 46.2%, P ϭ .151; transverse ligament 24.2% versus 23.1%, P ϭ 1.000). -
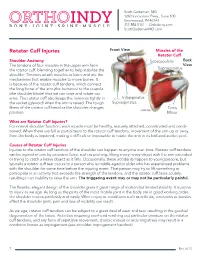
Rotator Cuff Injuries
Scott Gudeman, MD 1260 Innovation Pkwy., Suite 100 Greenwood, IN 46143 317.884.5161 OrthoIndy.com ScottGudemanMD.com Rotator Cuff Injuries Front View Muscles of the Rotator Cuff Shoulder Anatomy Subscapularis Back The tendons of four muscles in the upper arm form View Supraspinatus the rotator cuff, blending together to help stabilize the shoulder. Tendons attach muscles to bone and are the mechanisms that enable muscles to move bones. It is because of the rotator cuff tendons, which connect the long bone of the arm (the humerus) to the scapula (the shoulder blade) that we can raise and rotate our arms. The rotator cuff also keeps the humerus tightly in Infraspinatus the socket (glenoid) when the arm is raised. The tough Supraspinatus fibers of the rotator cuff bend as the shoulder changes Teres position. Minor What are Rotator Cuff Injuries? For normal shoulder function, each muscle must be healthy, securely attached, coordinated and condi- tioned. When there are full or partial tears to the rotator cuff tendons, movement of the arm up or away from the body is impaired, making it difficult or impossible to rotate the arm in its ball-and-socket joint. Causes of Rotator Cuff Injuries Injuries to the rotator cuff tendons of the shoulder can happen to anyone over time. Rotator cuff tendons can be injured or torn by excessive force, such as pitching, lifting a very heavy object with the arm extended or trying to catch a heavy object as it falls. Occasionally, these accidents happen to young people, but typically a rotator cuff tear occurs to a person who is middle-aged or older who has experienced problems with the shoulder for some time before the injuring event. -

Common Elbow Injuries Symptoms
During the summer months, many people stay active by playing golf or tennis. These sports, however, carry a risk of injury to the tendons – bands of tissue that connect muscles to bones – in the elbow. This month’s AT Corner will explain how these injuries happen, how to treat them if they occur and, most importantly, how to prevent them. Common Elbow Injuries Tendonitis: Inflammation, pain and difficulty using the joint caused by repetitive activities and/or sudden trauma. Tendonosis: A degeneration (breakdown) or tear of tendons which occurs as a result of aging. Symptoms of tendonosis usually last more than a few weeks. Note: Your risk of tendonitis and tendonosis increases with age. They also occur more frequently in those who routinely perform activities that require repetitive movement, as this places greater amounts of stress on the tendons. Tennis elbow: Also referred to as lateral epicondylitis, this condition occurs when there is an injury to the outer elbow tendon. Golfers’ elbow: Also referred to as medial epicondylitis, this condition occurs when there is an injury to the inner elbow tendon. Note: Injuries to these tendons can occur in other sports and activities that use the wrist and forearm muscles. Most times, the dominant arm is the one affected. Symptoms • Pain that spreads from the elbow into the upper arm or down the forearm • Forearm weakness • Pain that can begin suddenly or gradually worsen over time • Difficulty with activities that require arm strength Treatment Over-the-counter medications: NSAIDs, such as ibuprofen (Advil®, Motrin®) and naproxen (Aleve®), or acetaminophen (Tylenol®) can provide pain relief.