Sebaceous Carcinoma Epidemiology and Genetics: Emerging Concepts and Clinical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sample Research Poster
Surgical management and lymph node biopsy of rare malignant cutaneous adnexal carcinomas: a population-based analysis of 7591 patients Amrita Goyal MD, 1 Theodore Marghitu,2 Nikhil Goyal BS,3 Nathan Rubin MS,4 Krishnan Patel MD,6 Kavita Goyal MD,1 Daniel O’Leary MD,5 Kimberly Bohjanen MD, 1 Ian Maher MD 1 1Department of Dermatology, University of Minnesota, Minneapolis, MN 2University of Minnesota Medical School, Minneapolis, MN 3National Institutes of Health/National Cancer Institute, Bethesda, MD 4Biostatistics Core, Masonic Cancer Center, University of Minnesota, Minneapolis MN 5Division of Hematology, Oncology, and Transplantation, Department of Medicine, University of Minnesota, Minneapolis, MN 6Department of Radiation Oncology, University of Minnesota, Minneapolis, MN Background Overall and Disease-Specific Survival Lymph Node Biopsy and Survival Cutaneous adnexal carcinomas comprise a group of Vital status* All Sweat Hidradenocarc Spiradenocarci Sclerosin Porocarcin Eccrine Sebaceous Lymph Nodes All adnexal tumors adnexal gland inoma noma g sweat oma adenocarci carcinoma Lymph Nodes Examined carcino duct noma Nodes not examined 6592 (91.9) rare cutaneous malignancies that are generally ma tumor Nodes examined 578 (8.1) (MAC) Positive (% of examined) 138 (23.9) considered non-aggressive. Guidelines for the Stage (Derived AJCC N=1863 N=70 N=127 N=46 N=236 N=229 N=187 N=968 Negative (% of examined) 440 (76.1) Stage Group, 6th ed treatment of many of these malignancies are sparse, (2004-2015) Total N=1221 5-year OS 5-year DSS 1,2 I 1221 40 (57.1) 56 (44.1) 14 (30.4) 150 140 (61.1) 103 (55.1) 718 (74.2) Stage I Examined N=112 including guidance on surgical management (65.5) (63.6) Nodes not examined (% of total) 1109 (90.8) 69.7 (66.1-72.4) 99.3 (99.6-100) 3,4 II 440 14 (20.0) 54 (47.5) 28 (60.9) 47 (19.9) 64 (27.9) 51 (27.3) 182 (18.8) Nodes positive (% of examined) 0 (0) -- -- including the utility of lymph node biopsy. -

A Rare Clinical Presentation of Desmoplastic Trichilemmoma
Revista5Vol89ingles_Layout 1 8/8/14 10:17 AM Página 796 796 CASE REPORT s A rare clinical presentation of Desmoplastic Trichilemmoma mimicking Invasive Carcinoma* Daniela Tiemi Sano1 Jeane Jeong Hoon Yang1 Antonio José Tebcherani1 Luiz Arthur de Paula Machado Bazzo1 DOI: http://dx.doi.org/10.1590/abd1806-4841.20143095 Abstract: Trichilemmoma is a benign neoplasm from the outer sheath of the pilosebaceous follicle. Desmoplastic trichilemmoma, a rare variant, is histologically characterized by a central area of desmoplasia that can clinically mimic an invasive carcinoma, requiring histopathological examination to define the diagnosis. Keywords: Hair diseases; Hair follicle; Skin neoplasms INTRODUCTION The trichilemmoma is a benign solid tumor ori- ma, without the presence of malignant processes, and ginating from external sheath cells of pilosebaceous associated with nevus sebaceous of Jadassohn in the follicles, and the desmoplastic trichilemmoma is a rare periphery of the lesion (Figures 3, 4, 5 and 6). Patient benign histological variant.1,2,3 Clinically, it may look is still under outpatient follow-up, with good clinical like other cutaneous lesions.2 Among the differential evolution and no relapse of lesion. diagnoses, we can cite basal-cell carcinoma, squamous cell carcinoma and viral lesions; the histopathological DISCUSSION examination is necessary for diagnostic confirmation. The trichilemmoma is a benign tumor origina- We report here a case of desmoplastic trichilemmoma ting from external root sheath cells of pilosebaceous in a -

Inherited Skin Tumour Syndromes
CME GENETICS Clinical Medicine 2017 Vol 17, No 6: 562–7 I n h e r i t e d s k i n t u m o u r s y n d r o m e s A u t h o r s : S a r a h B r o w n , A P a u l B r e n n a n B a n d N e i l R a j a n C This article provides an overview of selected genetic skin con- and upper trunk. 1,2 These lesions are fibrofolliculomas, ditions where multiple inherited cutaneous tumours are a cen- trichodiscomas and acrochordons. Patients are also susceptible tral feature. Skin tumours that arise from skin structures such to the development of renal cell carcinoma, lung cysts and as hair, sweat glands and sebaceous glands are called skin pneumothoraces. 3 appendage tumours. These tumours are uncommon, but can Fibrofolliculomas and trichodiscomas clinically present as ABSTRACT have important implications for patient care. Certain appenda- skin/yellow-white coloured dome shaped papules 2–4 mm in geal tumours, particularly when multiple lesions are seen, may diameter (Fig 1 a and Fig 1 b). 4 These lesions usually develop indicate an underlying genetic condition. These tumours may in the third or fourth decade.4 In the case of fibrofolliculoma, not display clinical features that allow a secure diagnosis to be hair specific differentiation is seen, whereas in the case of made, necessitating biopsy and dermatopathological assess- trichodiscoma, differentiation is to the mesodermal component ment. -

Genetics of Skin Appendage Neoplasms and Related Syndromes
811 J Med Genet: first published as 10.1136/jmg.2004.025577 on 4 November 2005. Downloaded from REVIEW Genetics of skin appendage neoplasms and related syndromes D A Lee, M E Grossman, P Schneiderman, J T Celebi ............................................................................................................................... J Med Genet 2005;42:811–819. doi: 10.1136/jmg.2004.025577 In the past decade the molecular basis of many inherited tumours in various organ systems such as the breast, thyroid, and endometrium.2 syndromes has been unravelled. This article reviews the clinical and genetic aspects of inherited syndromes that are Clinical features of Cowden syndrome characterised by skin appendage neoplasms, including The cutaneous findings of Cowden syndrome Cowden syndrome, Birt–Hogg–Dube syndrome, naevoid include trichilemmomas, oral papillomas, and acral and palmoplantar keratoses. The cutaneous basal cell carcinoma syndrome, generalised basaloid hallmark of the disease is multiple trichilemmo- follicular hamartoma syndrome, Bazex syndrome, Brooke– mas which present clinically as rough hyperker- Spiegler syndrome, familial cylindromatosis, multiple atotic papules typically localised on the face (nasolabial folds, nose, upper lip, forehead, ears3 familial trichoepitheliomas, and Muir–Torre syndrome. (fig 1A, 1C, 1D). Trichilemmomas are benign ........................................................................... skin appendage tumours or hamartomas that show differentiation towards the hair follicles kin consists of both epidermal and dermal (specifically for the infundibulum of the hair 4 components. The epidermis is a stratified follicle). Oral papillomas clinically give the lips, Ssquamous epithelium that rests on top of a gingiva, and tongue a ‘‘cobblestone’’ appearance basement membrane, which separates it and its and histopathologically show features of 3 appendages from the underlying mesenchymally fibroma. The mucocutaneous manifestations of derived dermis. -

Sebaceous Cell Carcinoma: a Persistent Challenge in Clinical And
erimenta xp l D E e r & m l a a t c o i l n o i Journal of Clinical & Experimental l g y C f R o e l ISSN: 2155-9554 s a e n Miyamoto et al., J Clin Exp Dermatol Res 2016, 7:3 a r r u c o h J Dermatology Research DOI: 10.4172/2155-9554.1000353 Review Article Open Access Sebaceous Cell Carcinoma: A Persistent Challenge in Clinical and Histopathological Diagnosis Denise Miyamoto1*, Beatrice Wang2, Cristina Miyamoto3,4, Valeria Aoki1, Li Anne Lim4, Paula Blanco4 and Miguel N Burnier4 1Department of Dermatology, University of São Paulo Medical School, Brazil 2Department of Dermatology, McGill University, Canada 3Department of Ophthalmology, Federal University of São Paulo, Brazil 4From the Henry C. Witelson Ocular Pathology Laboratory, McGill University, Canada *Corresponding author: Denise Miyamoto, University of São Paulo Medical School, São Paulo-State of São Paulo, Brazil, Tel: 514-934-76129; E-mail: [email protected] Received date: April 25, 2016; Accepted date: May 20, 2016; Published date: May 25, 2016 Copyright: © 2016 Miyamoto D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Sebaceous cell carcinoma continues to defy clinicians and pathologists in terms of early diagnosis. The tumor may be mistaken as benign lesions such as chalazion and blepharitis, and also as malignant neoplasms, mainly basal cell carcinoma and squamous cell carcinoma. Despite advances in immunohistochemical analysis and treatment options during the last decades, morbidity and metastasis rates remain high. -
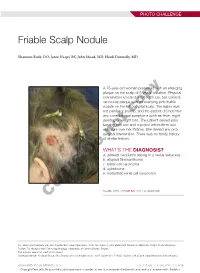
Friable Scalp Nodule
PHOTO CHALLENGE Friable Scalp Nodule Shannon Buck, DO; Jaree Naqvi, BS; John Moad, MD; Heidi Donnelly, MD A 75-year-old woman presented with an enlarging plaque on the scalp of 5 years’ duration. Physical examination revealed a 5.6×2.9-cm, tan-colored, verrucous plaque with an overlying pink friable nodule on the left occipital scalp. The lesion was not painful or pruritic,copy and the patient did not have any constitutional symptoms such as fever, night sweats, or weight loss. The patient denied prior tanning bed use and reported intermittent sun exposure over her lifetime. She denied any prior surgicalnot intervention. There was no family history of similar lesions. WHAT’S THE DIAGNOSIS? Doa. adnexal neoplasm arising in a nevus sebaceus b. atypical fibroxanthoma c. basal cell carcinoma d. cylindroma e. metastatic renal cell carcinoma CUTIS PLEASE TURN TO PAGE E20 FOR THE DIAGNOSIS Drs. Buck and Donnelly are from Dayton Skin Care Specialists, Ohio. Mr. Naqvi is from Boonshoft School of Medicine, Wright State University, Dayton. Dr. Moad is from Dermatopathology Laboratory of Central States, Dayton. The authors report no conflict of interest. Correspondence: Shannon Buck, DO, Dayton Skin Care Specialists, 3025 Governor’s Pl Blvd, Dayton, OH 45409 ([email protected]). WWW.MDEDGE.COM/DERMATOLOGY VOL. 105 NO. 1 I JANUARY 2020 E19 Copyright Cutis 2020. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. PHOTO CHALLENGE DISCUSSION THE DIAGNOSIS: Adnexal Neoplasm Arising in a Nevus Sebaceus iopsy of the lesion showed a proliferation of basa- secondary neoplasms, 88% of which were benign.2 The loid-appearing cells with focal ductal differentiation origins of these neoplasms can be epithelial, sebaceous, Band ulceration consistent with poroma (Figure 1). -

Sebaceous Carcinoma: Clinicopathologic Analysis of 29 Cases in a Tertiary Hospital in Korea
CROSSMARK_logo_3_Test 1 / 1 ORIGINAL ARTICLE Dermatology https://crossmarhttps://doi.org/10.3346/jkms.2017.32.8.1351k-cdn.crossref.org/widget/v2.0/logos/CROSSMARK_Color_square.svg 2017-03-16 • J Korean Med Sci 2017; 32: 1351-1359 Sebaceous Carcinoma: Clinicopathologic Analysis of 29 Cases in a Tertiary Hospital in Korea Su-Kyung Park,1 Jin Park,1,2 Sebaceous carcinoma (SC) is a neoplasm derived from the adnexal epithelium of the Han-Uk Kim,1,2 and Seok-Kweon Yun1,2 sebaceous glands, and most studies on this neoplasm have been conducted in Caucasians. We retrospectively reviewed the records of 29 patients with SC (16 extraocular and 13 1Department of Dermatology, Chonbuk National University Hospital, Jeonju, Korea; 2Research ocular lesions) who were diagnosed from 2001 to 2014 to analyze the clinical and Institute of Clinical Medicine of Chonbuk National histopathological features of SC in the Korean population. Sixteen of the patients were University, Biomedical Research Institute of women and 13 were men. There was an equal sex distribution for extraocular lesions, and Chonbuk National University Hospital, Jeonju, Korea a female predilection (M:F = 1:1.6) for ocular lesions. The mean ages at presentation of ± ± Received: 7 February 2017 extraocular and ocular lesions were 69.19 37.19 (range, 32–87) and 67.46 24.46 Accepted: 13 May 2017 (range, 43–85) years, respectively. Most lesions occurred in the eyelid (13/29, 44.83%), and most extraocular lesions occurred in the head and neck area (13/16, 81.25%). There Address for Correspondence: was no recurrence or death during the follow-up period. -

Biomarkers in Sebaceous Gland Carcinomas
3/24/2017 Biomarkers in Sebaceous Gland Carcinomas Sander R. Dubovy, MD Professor of Ophthalmology and Pathology Victor T. Curtin Chair in Ophthalmology Florida Lions Ocular Pathology Laboratory Bascom Palmer Eye Institute University of Miami Miller School of Medicine Biomarkers in Sebaceous Gland Carcinomas Disclosure of Relevant Disclosure of Relevant Financial Relationships Financial Relationships USCAP requires that all planners (Education Committee) in a position to Dr. Sander R. Dubovy declares he has no conflict(s) of interest influence or control the content of CME disclose any relevant financial to disclose. relationship WITH COMMERCIAL INTERESTS which they or their spouse/partner have, or have had, within the past 12 months, which relates to the content of this educational activity and creates a conflict of interest. Biomarkers in Sebaceous Gland Carcinomas Outline Introduction • Sebaceous carcinoma (SC) is a malignant neoplasm that arises from • Introduction to sebaceous cell carcinoma the sebaceous glands, most commonly in the periocular areas. • Incidence, demographics, risk factors • Clinical manifestations are often mistaken for benign conditions and • Ocular origins thus proper diagnosis and management is delayed. • Gross pathology • Metastases to regional lymph nodes and other sites are common. • Microscopic pathology • Immunohistochemistry • Management • Cases Biomarkers in Sebaceous Gland Carcinomas Biomarkers in Sebaceous Gland Carcinomas 1 3/24/2017 Introduction Sebaceous Gland • Pathologists should be aware of the -

Current Diagnosis and Treatment Options for Cutaneous Adnexal Neoplasms with Apocrine and Eccrine Differentiation
International Journal of Molecular Sciences Review Current Diagnosis and Treatment Options for Cutaneous Adnexal Neoplasms with Apocrine and Eccrine Differentiation Iga Płachta 1,2,† , Marcin Kleibert 1,2,† , Anna M. Czarnecka 1,* , Mateusz Spałek 1 , Anna Szumera-Cie´ckiewicz 3,4 and Piotr Rutkowski 1 1 Department of Soft Tissue/Bone Sarcoma and Melanoma, Maria Sklodowska-Curie National Research Institute of Oncology, 02-781 Warsaw, Poland; [email protected] (I.P.); [email protected] (M.K.); [email protected] (M.S.); [email protected] (P.R.) 2 Faculty of Medicine, Medical University of Warsaw, 02-091 Warsaw, Poland 3 Department of Pathology and Laboratory Diagnostics, Maria Sklodowska-Curie National Research Institute of Oncology, 02-781 Warsaw, Poland; [email protected] 4 Department of Diagnostic Hematology, Institute of Hematology and Transfusion Medicine, 00-791 Warsaw, Poland * Correspondence: [email protected] or [email protected] † Equally contributed to the work. Abstract: Adnexal tumors of the skin are a rare group of benign and malignant neoplasms that exhibit morphological differentiation toward one or more of the adnexal epithelium types present in normal skin. Tumors deriving from apocrine or eccrine glands are highly heterogeneous and represent various histological entities. Macroscopic and dermatoscopic features of these tumors are unspecific; therefore, a specialized pathological examination is required to correctly diagnose patients. Limited Citation: Płachta, I.; Kleibert, M.; treatment guidelines of adnexal tumor cases are available; thus, therapy is still challenging. Patients Czarnecka, A.M.; Spałek, M.; should be referred to high-volume skin cancer centers to receive an appropriate multidisciplinary Szumera-Cie´ckiewicz,A.; Rutkowski, treatment, affecting their outcome. -

A Clinico-Histopathological Study on Skin Appendageal Tumors Paudyal P1, Agarwal M1, Pradhan A1, Sinha AK1, Agrawal S 2
Journal of Pathology of Nepal (2016) Vol. 6, 885 - 891 Journal of cal Patholo lini gis f C t o o f N n e io p t a a l i - c 2 PATHOLOGY o 0 s 1 s 0 A N u e d p n of Nepal a l a M m e h d t i a c K al , A ad ss o oc n R www.acpnepal.com iatio bitio n Building Exhi Original Article A clinico-histopathological study on skin appendageal tumors Paudyal P1, Agarwal M1, Pradhan A1, Sinha AK1, Agrawal S 2 1Department of Pathology, B P Koirala Institute of Health Sciences, Dharan, Nepal. 2Department of Dermatology and Venerology, B P Koirala Institute of Health Sciences, Dharan, Nepal. ABSTRACT Keywords: Background: Skin Appendageal tumors are a large and diverse group of tumors that are commonly Skin adnexal tumor; Pilomatricoma; classiied according to their state of appendageal differentiation: follicular, sebaceous, eccrine and Keratoacanthoma apocrine. Objectives of this study were to study the clinic-epidemiological proile of skin appendageal tumors and to correlate the clinico-histopathological diagnosis. Materials and Methods: This was a retrospective and prospective study which included all cases of skin adnexal tumours diagnosed histologically during the period of four and half years. Tumors were analyzed considering the anatomic location, duration, size and type of the tumour, along with age and sex of the patient. The Histological characterization was done according to the WHO classiication system and was correlated with the clinical diagnosis. Collected data were analysed using SPSS PC+ 11.5 Version for statistical analysis. -

Revista6vol86v6 ING Layout 1
1213 IMAGENS EM DERMATOLOGIA ▲ Aspectos dermatoscópicos do siringocistoadenoma papilífero associado a nevo sebáceo * Dermoscopic aspects of syringocystadenoma papilliferum associated with nevus sebaceus Carolina Barbosa Bruno1 Fernanda Nóbrega Cordeiro1 Fernando do Espírito Santo Soares2 Gustavo Henrique Soares Takano3 Larissa Sena Teixeira Mendes4 Resumo: O siringocistoadenoma papilífero é uma neoplasia anexial benigna rara, com frequente dife- renciação apócrina. Localiza-se preferencialmente no couro cabeludo e está associado ao nevo sebáceo em 40% dos casos. Apesar da variabilidade clínica, a histologia é característica. Há relatos da dermatos- copia de tumores anexiais, como poroma écrino, hidradenoma e angio-histiocitoma; porém, até o momento, não há descrição da dermatoscopia do siringocistoadenoma. Apresentamos aspectos derma- toscópicos de um caso de siringocistoadenoma associado a nevo sebáceo, visualizando-se padrão vascu- lar polimorfo e vasos em ferradura. Palavras-chave: Avaliação; Dermoscopia; Diagnóstico; Neoplasias de anexos e de apêndices cutâneos; Neoplasias cutâneas Abstract: Syringocystadenoma papilliferum is a rare benign adnexal tumor that frequently shows apo- crine differentiation. It usually develops on the scalp and is associated with a nevus sebaceus in 40% of cases. Although the clinical presentation may differ, its histology is characteristic. Reports have been made of dermoscopy used in cases of adnexal tumors such as eccrine poromas, hidradenomas and angiohistiocytomas; however, up to the present moment -

Areclinicianssuccessful in Diagnosingcutaneousadnexaltumors? Aretrospective, Clinicopathologicalstudy
Turkish Journal of Medical Sciences Turk J Med Sci (2020) 50: 832-843 http://journals.tubitak.gov.tr/medical/ © TÜBİTAK Research Article doi:10.3906/sag-2002-126 Areclinicianssuccessful in diagnosingcutaneousadnexaltumors? aretrospective, clinicopathologicalstudy 1, 1 1 Melek ASLAN KAYIRAN *, Ayşe Serap KARADAĞ , Yasin KÜÇÜK , 2 1 1 Bengü ÇOBANOĞLU ŞİMŞEK , Vefa Aslı ERDEMİR , Necmettin AKDENİZ 1 Department of Dermatology, Göztepe Training and Research Hospital, İstanbul Medeniyet University, İstanbul, Turkey 2 Department of Pathology, Göztepe Training and Research Hospital, İstanbul Medeniyet University, İstanbul, Turkey Received: 15.02.2020 Accepted/Published Online: 11.04.2020 Final Version: 23.06.2020 Background/aim: Cutaneous adnexal tumors (CAT) are rare tumors originating from the adnexal epithelial parts of the skin. Due to its clinical and histopathological characteristics comparable with other diseases, clinicians and pathologists experience difficulties in its diagnosis. We aimed to reveal the clinical and histopathological characteristics of the retrospectively screened cases and to compare the prediagnoses and histopathological diagnoses of clinicians. Materials and methods: The data of the last 5 years were scanned and patients with histopathological diagnosis of CAT were included in the study. Results: A total of 65 patients, including 39 female and 26 male patients aged between 8 and 88, were included in the study. The female to male ratio was 1.5, and the mean age of the patients was 46.15 ± 21.8 years. The benign tumor rate was 95.4%, whereas the malignant tumor rate was 4.6%. 38.5% of the tumors were presenting sebaceous, 35.4% of them were presenting follicular, and 18.5% of them were presenting eccrine differentiation.