Sample Research Poster
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Atypical Compound Nevus Arising in Mature Cystic Ovarian Teratoma
J Cutan Pathol 2005: 32: 71–123 Copyright # Blackwell Munksgaard 2005 Blackwell Munksgaard. Printed in Denmark Journal of Cutaneous Pathology Abstracts of the Papers Presented at the 41st Annual Meeting of The American Society of Dermatopathology Westin Copley Place Boston, Massachusetts, USA October 14–17, 2004 These abstracts were presented in oral or poster format at the 41st Annual Meeting of The American Society of Dermatopathology on October 14–17, 2004. They are listed on the following pages in alphabetical order by the first author’s last name. 71 Abstracts IN SITU HYBRIDIZATION IS A VALUABLE DIAGNOSTIC A 37-year-old woman with diagnosis of Sjogren’s syndrome (SS) TOOL IN CUTANEOUS DEEP FUNGAL INFECTIONS presented with asymptomatic non-palpable purpura of the lower J.J. Abbott1, K.L. Hamacher2,A.G.Bridges2 and I. Ahmed1,2 extremities. Biopsy of a purpuric macule revealed a perivascular Departments of Laboratory Medicine and Pathology1 and and focally nodular lymphocytic infiltrate with large numbers of Dermatology2, plasma cells, seemingly around eccrine glands. There was no vascu- litis. The histologic findings in the skin were strikingly similar to those Mayo Clinic and Mayo Foundation, Rochester, MN, USA of salivary, parotid, and other ‘‘secretory’’ glands affected in SS. The cutaneous manifestations of SS highlighted in textbooks include Dimorphic fungal infections (histoplasmosis, blastomycosis, coccidiomy- xerosis, annular erythema, small-vessel vasculitis, and pigmented cosis, and cryptococcosis) can occur in immunocompromised and purpura. This case illustrates that purpura in skin of patients with healthy individuals. Cutaneous involvement is often secondary and SS may be caused by a peri-eccrine plasma-rich infiltrate. -

Malignant Hidradenoma: a Report of Two Cases and Review of the Literature
ANTICANCER RESEARCH 26: 2217-2220 (2006) Malignant Hidradenoma: A Report of Two Cases and Review of the Literature I.E. LIAPAKIS1, D.P. KORKOLIS2, A. KOUTSOUMBI3, A. FIDA3, G. KOKKALIS1 and P.P. VASSILOPOULOS2 1Department of Plastic and Reconstructive Surgery, 2First Department of Surgical Oncology and 3Department of Surgical Pathology, Hellenic Anticancer Institute, "Saint Savvas" Hospital, Athens, Greece Abstract. Introduction: Malignant tumors of the sweat glands difficult (1). Clear cell hidradenoma is an extremely rare are very rare. Clear cell hidradenoma is a lesion with tumor with less than 50 cases reported (2, 3). histopathological features resembling those of eccrine poroma The cases of two patients, suffering from aggressive and eccrine spiradenoma. The biological behavior of the tumor dermal lesions invading the abdominal wall and the axillary is aggressive, with local recurrences reported in more than 50% region, are described here. Surgical resection and of the surgically-treated cases. Materials and Methods: Two histopathological examination ascertained the presence of patients are presented, the first with tumor in the right axillary malignant clear cell hidradenoma. In addition to these region, the second with a recurrent tumor of the abdominal cases, a review of the literature is also presented. wall. The first patient underwent wide excision with clear margins and axillary lymph node dissection and the second Case Reports patient underwent wide excision of the primary lesion and bilateral inguinal node dissection due to palpable nodes. Patient 1. Patient 1 was a 68-year-old Caucasian male who had Results: The patients had uneventful postoperative courses. No undergone excision of a rapidly growing, ulcerous lesion of the additional treatment was administered. -

Malignant Nodular Hidradenoma-Inguinal Region Clinically Masquerading As Squamous Cell Carcinoma: a Case Report
International Journal of Research in Medical Sciences Vernekar S et al. Int J Res Med Sci. 2019 Jul;7(7):2848-2852 www.msjonline.org pISSN 2320-6071 | eISSN 2320-6012 DOI: http://dx.doi.org/10.18203/2320-6012.ijrms20192933 Case Report Malignant nodular hidradenoma-inguinal region clinically masquerading as squamous cell carcinoma: a case report Sunita S. Vernekar, Priyadharshini Bargunam* Department of Pathology, Karnataka Institute of Medical Sciences, Hubballi, Karnataka, India Received: 24 April 2019 Accepted: 05 June 2019 *Correspondence: Dr. Priyadharshini Bargunam, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Malignant Nodular hidradenoma is an extremely rare aggressive tumour originating from eccrine sweat glands with an incidence of <.001%. So far less than 80 cases have been reported in the literature. It’s known for its local recurrence (50%) and metastasis (60%) and hence early diagnosis and radical treatment is mandatory. But differentiating it from its benign counterparts and other skin tumour mimics is challenging, due to its histopathological similarity & lack of diagnostic immunomarkers. Authors report a case of 65-year-old female who presented with a short 4-month history of rapidly growing ulceroproliferative growth in the right inguinal region with bilateral inguinal node enlargement, associated with pain and discharge. Wedge biopsy of left inguinal lymph node showed malignant cutaneous adnexal tumour deposits, which after excision was typed as malignant nodular hidradenoma. -

A Rare Clinical Presentation of Desmoplastic Trichilemmoma
Revista5Vol89ingles_Layout 1 8/8/14 10:17 AM Página 796 796 CASE REPORT s A rare clinical presentation of Desmoplastic Trichilemmoma mimicking Invasive Carcinoma* Daniela Tiemi Sano1 Jeane Jeong Hoon Yang1 Antonio José Tebcherani1 Luiz Arthur de Paula Machado Bazzo1 DOI: http://dx.doi.org/10.1590/abd1806-4841.20143095 Abstract: Trichilemmoma is a benign neoplasm from the outer sheath of the pilosebaceous follicle. Desmoplastic trichilemmoma, a rare variant, is histologically characterized by a central area of desmoplasia that can clinically mimic an invasive carcinoma, requiring histopathological examination to define the diagnosis. Keywords: Hair diseases; Hair follicle; Skin neoplasms INTRODUCTION The trichilemmoma is a benign solid tumor ori- ma, without the presence of malignant processes, and ginating from external sheath cells of pilosebaceous associated with nevus sebaceous of Jadassohn in the follicles, and the desmoplastic trichilemmoma is a rare periphery of the lesion (Figures 3, 4, 5 and 6). Patient benign histological variant.1,2,3 Clinically, it may look is still under outpatient follow-up, with good clinical like other cutaneous lesions.2 Among the differential evolution and no relapse of lesion. diagnoses, we can cite basal-cell carcinoma, squamous cell carcinoma and viral lesions; the histopathological DISCUSSION examination is necessary for diagnostic confirmation. The trichilemmoma is a benign tumor origina- We report here a case of desmoplastic trichilemmoma ting from external root sheath cells of pilosebaceous in a -

Adnexal Tumors
10/24/2019 What’s a gland like you doing in a place like this? A practical approach to cutaneous adnexal neoplasms Hafeez Diwan, MD, PhD Departments of Pathology & Immunology and Dermatology Baylor College of Medicine 1 Conflict of interest • None 2 Disclosures • I have nothing to disclose 3 1 10/24/2019 Is the adnexal neoplasm glandular? And if so, where is it located? • Hands and Feet: Digital papillary adenocarcinoma 4 5 6 2 10/24/2019 7 8 Digital Papillary Adenocarcinoma • Solitary • Fingers/toes/palms/soles • Recurrence/metastases 9 3 10/24/2019 10 11 12 4 10/24/2019 3 Points about digital papillary adenocarcinoma • 1. Atypia doesn’t matter – if there is no atypia, it doesn’t mean that it isn’t digital papillary adenocarcinoma 13 3 Points about digital papillary adenocarcinoma • 1. Atypia doesn’t matter – if there is no atypia, it doesn’t mean that it isn’t digital papillary adenocarcinoma • 2. How high can the glandular lesion go up the extremity? • Example of one case that occurred on the thigh? (Alomari A, Douglas S, Galan A, Narayan D, Ko C. Atypical Presentation of digital papillary adenocarcinoma (abstract) J Cutan Pathol. 2014;41:221) 14 3 Points about digital papillary adenocarcinoma (cont’d) • 3. What if you don’t see glands • Hidradenoma on hands and feet • Hunt for a gland? If you see a gland, then what? • Probably best to err on the side of caution and say that a digital papillary adenocarcinoma is not ruled out 15 5 10/24/2019 16 17 18 6 10/24/2019 19 20 21 7 10/24/2019 3 Points about digital papillary adenocarcinoma (cont’d) • 3. -

Inherited Skin Tumour Syndromes
CME GENETICS Clinical Medicine 2017 Vol 17, No 6: 562–7 I n h e r i t e d s k i n t u m o u r s y n d r o m e s A u t h o r s : S a r a h B r o w n , A P a u l B r e n n a n B a n d N e i l R a j a n C This article provides an overview of selected genetic skin con- and upper trunk. 1,2 These lesions are fibrofolliculomas, ditions where multiple inherited cutaneous tumours are a cen- trichodiscomas and acrochordons. Patients are also susceptible tral feature. Skin tumours that arise from skin structures such to the development of renal cell carcinoma, lung cysts and as hair, sweat glands and sebaceous glands are called skin pneumothoraces. 3 appendage tumours. These tumours are uncommon, but can Fibrofolliculomas and trichodiscomas clinically present as ABSTRACT have important implications for patient care. Certain appenda- skin/yellow-white coloured dome shaped papules 2–4 mm in geal tumours, particularly when multiple lesions are seen, may diameter (Fig 1 a and Fig 1 b). 4 These lesions usually develop indicate an underlying genetic condition. These tumours may in the third or fourth decade.4 In the case of fibrofolliculoma, not display clinical features that allow a secure diagnosis to be hair specific differentiation is seen, whereas in the case of made, necessitating biopsy and dermatopathological assess- trichodiscoma, differentiation is to the mesodermal component ment. -

Genetics of Skin Appendage Neoplasms and Related Syndromes
811 J Med Genet: first published as 10.1136/jmg.2004.025577 on 4 November 2005. Downloaded from REVIEW Genetics of skin appendage neoplasms and related syndromes D A Lee, M E Grossman, P Schneiderman, J T Celebi ............................................................................................................................... J Med Genet 2005;42:811–819. doi: 10.1136/jmg.2004.025577 In the past decade the molecular basis of many inherited tumours in various organ systems such as the breast, thyroid, and endometrium.2 syndromes has been unravelled. This article reviews the clinical and genetic aspects of inherited syndromes that are Clinical features of Cowden syndrome characterised by skin appendage neoplasms, including The cutaneous findings of Cowden syndrome Cowden syndrome, Birt–Hogg–Dube syndrome, naevoid include trichilemmomas, oral papillomas, and acral and palmoplantar keratoses. The cutaneous basal cell carcinoma syndrome, generalised basaloid hallmark of the disease is multiple trichilemmo- follicular hamartoma syndrome, Bazex syndrome, Brooke– mas which present clinically as rough hyperker- Spiegler syndrome, familial cylindromatosis, multiple atotic papules typically localised on the face (nasolabial folds, nose, upper lip, forehead, ears3 familial trichoepitheliomas, and Muir–Torre syndrome. (fig 1A, 1C, 1D). Trichilemmomas are benign ........................................................................... skin appendage tumours or hamartomas that show differentiation towards the hair follicles kin consists of both epidermal and dermal (specifically for the infundibulum of the hair 4 components. The epidermis is a stratified follicle). Oral papillomas clinically give the lips, Ssquamous epithelium that rests on top of a gingiva, and tongue a ‘‘cobblestone’’ appearance basement membrane, which separates it and its and histopathologically show features of 3 appendages from the underlying mesenchymally fibroma. The mucocutaneous manifestations of derived dermis. -

Malignant Nodular Hidradenocarcinoma Arising on The
genesi ino s & rc a M C u t f a o g l Journal of Carcinogenesis & e Giorgini et al., J Carcinogene Mutagene 2012, 3:1 a n n e r s DOI: 4172/2157-2518.1000129 u i s o J Mutagenesis ISSN: 2157-2518 ReviewResearch Article Article OpenOpen Access Access Malignant Nodular Hidradenocarcinoma Arising on the Areola of a Male Patient: Case Report of an “Orphan Disease” and Review of the Literature Eleonora Giorgini1*, Gregorio Tugnoli1, Silvia Aprile1, Guido Collina2, Silvia Villani1, Andrea Biscardi1,Simone Maggioli1, Eli Avisar3 and Salomone Di Saverio1 1Department of Emergency & Surgery, Maggiore Hospital, Bologna Local Health District Largo Nigrisoli 2, 40100 Bologna, Emergency Surgery and Trauma Surgery Unit, Italy 2Department of Pathology, Maggiore Hospital, Bologna Local Health District Largo Nigrisoli 2, 40100 Bologna, Italy 3Department of Surgery, Sylvester Comprehensive Cancer Center, Miller School of Medicine, University of Miami, UM/SCCC 1475 NW 12th Avenue, Rm, 3550, Miami, FL ZIP 33136, USA Abstract Herein we describe a rare case of an “orphan” neoplasm arising on an unusual site. During the clinical examination, a mass of 3 cm was found on the left areola of a male patient. It was a solid, sliding on the deep layer, with a bluish color mass; therefore an excisional biopsy was performed. The histopathological diagnosis was nodular malignant hidradenoma. An oncological consulting recommended a surgical radicalization through a radical mastectomy. No adjuvant therapy has been given. The patient is alive with no evidence of disease after one-year follow up. Keywords: Male breast cancer; Eccrine gland-derived carcinoma; highly vascularized. -

Sebaceous Cell Carcinoma: a Persistent Challenge in Clinical And
erimenta xp l D E e r & m l a a t c o i l n o i Journal of Clinical & Experimental l g y C f R o e l ISSN: 2155-9554 s a e n Miyamoto et al., J Clin Exp Dermatol Res 2016, 7:3 a r r u c o h J Dermatology Research DOI: 10.4172/2155-9554.1000353 Review Article Open Access Sebaceous Cell Carcinoma: A Persistent Challenge in Clinical and Histopathological Diagnosis Denise Miyamoto1*, Beatrice Wang2, Cristina Miyamoto3,4, Valeria Aoki1, Li Anne Lim4, Paula Blanco4 and Miguel N Burnier4 1Department of Dermatology, University of São Paulo Medical School, Brazil 2Department of Dermatology, McGill University, Canada 3Department of Ophthalmology, Federal University of São Paulo, Brazil 4From the Henry C. Witelson Ocular Pathology Laboratory, McGill University, Canada *Corresponding author: Denise Miyamoto, University of São Paulo Medical School, São Paulo-State of São Paulo, Brazil, Tel: 514-934-76129; E-mail: [email protected] Received date: April 25, 2016; Accepted date: May 20, 2016; Published date: May 25, 2016 Copyright: © 2016 Miyamoto D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Sebaceous cell carcinoma continues to defy clinicians and pathologists in terms of early diagnosis. The tumor may be mistaken as benign lesions such as chalazion and blepharitis, and also as malignant neoplasms, mainly basal cell carcinoma and squamous cell carcinoma. Despite advances in immunohistochemical analysis and treatment options during the last decades, morbidity and metastasis rates remain high. -
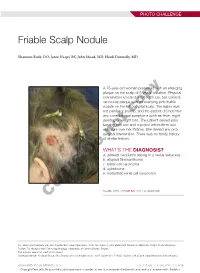
Friable Scalp Nodule
PHOTO CHALLENGE Friable Scalp Nodule Shannon Buck, DO; Jaree Naqvi, BS; John Moad, MD; Heidi Donnelly, MD A 75-year-old woman presented with an enlarging plaque on the scalp of 5 years’ duration. Physical examination revealed a 5.6×2.9-cm, tan-colored, verrucous plaque with an overlying pink friable nodule on the left occipital scalp. The lesion was not painful or pruritic,copy and the patient did not have any constitutional symptoms such as fever, night sweats, or weight loss. The patient denied prior tanning bed use and reported intermittent sun exposure over her lifetime. She denied any prior surgicalnot intervention. There was no family history of similar lesions. WHAT’S THE DIAGNOSIS? Doa. adnexal neoplasm arising in a nevus sebaceus b. atypical fibroxanthoma c. basal cell carcinoma d. cylindroma e. metastatic renal cell carcinoma CUTIS PLEASE TURN TO PAGE E20 FOR THE DIAGNOSIS Drs. Buck and Donnelly are from Dayton Skin Care Specialists, Ohio. Mr. Naqvi is from Boonshoft School of Medicine, Wright State University, Dayton. Dr. Moad is from Dermatopathology Laboratory of Central States, Dayton. The authors report no conflict of interest. Correspondence: Shannon Buck, DO, Dayton Skin Care Specialists, 3025 Governor’s Pl Blvd, Dayton, OH 45409 ([email protected]). WWW.MDEDGE.COM/DERMATOLOGY VOL. 105 NO. 1 I JANUARY 2020 E19 Copyright Cutis 2020. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. PHOTO CHALLENGE DISCUSSION THE DIAGNOSIS: Adnexal Neoplasm Arising in a Nevus Sebaceus iopsy of the lesion showed a proliferation of basa- secondary neoplasms, 88% of which were benign.2 The loid-appearing cells with focal ductal differentiation origins of these neoplasms can be epithelial, sebaceous, Band ulceration consistent with poroma (Figure 1). -

Modpathol201046.Pdf
Modern Pathology (2010) 23, 713–719 & 2010 USCAP, Inc. All rights reserved 0893-3952/10 $32.00 713 The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin Meera Mahalingam1, Lisa P Nguyen1, Joanna E Richards1, Alona Muzikansky2 and Mai P Hoang3,4 1Dermatopathology Section, Department of Dermatology, Boston University School of Medicine, Boston, MA, USA; 2Biostatistics Center, Massachusetts General Hospital, Boston, MA, USA; 3Department of Pathology, Massachusetts General Hospital, Boston, MA, USA and 4Harvard Medical School, Boston, MA, USA Often the distinction of primary adnexal carcinoma from metastatic adenocarcinoma to skin from breast, lung, and other sites can be a diagnostic dilemma. Current markers purportedly of utility as diagnostic adjuncts include p63 and D2-40; however, their expression has been demonstrated in 11–22% and 5% of metastatic cutaneous metastases, respectively. Both cytokeratin (CK) 15 and nestin have been reported as follicular stem cell markers. We performed CK15 and nestin, as well as previously reported stains (such as p63, D2-40, and calretinin) on 113 cases (59 primary adnexal carcinomas and 54 cutaneous metastases). Expressions of p63, CK15, nestin, D2-40, and calretinin were observed in 91, 40, 37, 44, and 14% of primary adnexal carcinoma, respectively, and in 8, 2, 8, 4, and 10% of cutaneous metastases, respectively. p63 appeared to be the most sensitive marker (with a sensitivity of 91%) in detecting primary adnexal carcinomas. CK15 appeared to be the most specific marker with a specificity of 98%. Using v2 analysis, statistically significant P-values (o0.05) were observed for p63, CK15, nestin, and D2-40 in the distinction of primary adnexal carcinoma versus cutaneous metastases. -

A Rare Case of Trichilemmal Carcinoma: Histology and Management
A rare case of Trichilemmal Carcinoma: histology and management Lisa Fronek DO, Allyson Brahs BS, Maheera Farsi DO, Richard Miller DO, Dudith Pierre-Victor, PhD, MPH HCA Healthcare USF Morsani College of Medicine: Largo Medical Center Program Western University of Heath Sciences, College of Osteopathic Medicine of the Pacific Introduction Clinical and Histologic Findings Discussion Trichilemmal carcinoma (TC) is a rare, malignant, adnexal neoplasm that is TC is a rare, adnexal tumor with evidence for follicular ORS or trichilemmal derived from the outer root sheath (ORS) of the hair follicle. These tumors differentiation. It is considered the malignant analogue of trichilemmoma. predominantly occur in elderly patients on sun-exposed areas, specifically on Clinical presentation is variable; due to its ability to resemble different clinical the head and neck with the face defined as the most common location. The entities, the diagnosis of TC relies on histological evaluation, accompanied by mean age of diagnosis is 70 years old with a slight male predominance. IHC. Microscopically, TC features a solid, lobular, or trabecular growth pattern These lesions are commonly identified as a papular, nodular, and sometimes, often centered around a pilosebaceous unit. The tumor cells are clear, exophytic. They generally arise de-novo, but may also derivate from an polygonal, and glycogen-rich (periodic acid-Schiff positive (PAS), diastase underlying proliferating trichilemmal cyst with a loss of p53, a seborrheic sensitive), reminiscent of clear cells of the ORS. It exhibits peripheral keratosis, a nevus sebaceous, or a scar. They can be locally aggressive and palisading of basaloid cells abutting a sometimes thickened hyalinized may exhibit telangiectasias and ulceration due to local destruction.