Electrons in Condensed Matter ~"
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture Notes: BCS Theory of Superconductivity
Lecture Notes: BCS theory of superconductivity Prof. Rafael M. Fernandes Here we will discuss a new ground state of the interacting electron gas: the superconducting state. In this macroscopic quantum state, the electrons form coherent bound states called Cooper pairs, which dramatically change the macroscopic properties of the system, giving rise to perfect conductivity and perfect diamagnetism. We will mostly focus on conventional superconductors, where the Cooper pairs originate from a small attractive electron-electron interaction mediated by phonons. However, in the so- called unconventional superconductors - a topic of intense research in current solid state physics - the pairing can originate even from purely repulsive interactions. 1 Phenomenology Superconductivity was discovered by Kamerlingh-Onnes in 1911, when he was studying the transport properties of Hg (mercury) at low temperatures. He found that below the liquifying temperature of helium, at around 4:2 K, the resistivity of Hg would suddenly drop to zero. Although at the time there was not a well established model for the low-temperature behavior of transport in metals, the result was quite surprising, as the expectations were that the resistivity would either go to zero or diverge at T = 0, but not vanish at a finite temperature. In a metal the resistivity at low temperatures has a constant contribution from impurity scattering, a T 2 contribution from electron-electron scattering, and a T 5 contribution from phonon scattering. Thus, the vanishing of the resistivity at low temperatures is a clear indication of a new ground state. Another key property of the superconductor was discovered in 1933 by Meissner. -

Concepts of Condensed Matter Physics Spring 2015 Exercise #1
Concepts of condensed matter physics Spring 2015 Exercise #1 Due date: 21/04/2015 1. Adding long range hopping terms In class we have shown that at low energies (near half-filling) electrons in graphene have a doubly degenerate Dirac spectrum located at two points in the Brillouin zone. An important feature of this dispersion relation is the absence of an energy gap between the upper and lower bands. However, in our analysis we have restricted ourselves to the case of nearest neighbor hopping terms, and it is not clear if the above features survive the addition of more general terms. Write down the Bloch-Hamiltonian when next nearest neighbor and next-next nearest neighbor terms are included (with amplitudes 푡’ and 푡’’ respectively). Draw the spectrum for the case 푡 = 1, 푡′ = 0.4, 푡′′ = 0.2. Show that the Dirac cones survive the addition of higher order terms, and find the corresponding low-energy Hamiltonian. In the next question, you will study under which circumstances the Dirac cones remain stable. 2. The robustness of Dirac fermions in graphene –We know that the lattice structure of graphene has unique symmetries (e.g. 3-fold rotational symmetry of the honeycomb lattice). The question is: What protects the Dirac spectrum? Namely, what inherent symmetry in graphene we need to violate in order to destroy the massless Dirac spectrum of the electrons at low energies (i.e. open a band gap). In this question, consider only nearest neighbor terms. a. Stretching the graphene lattice - one way to reduce the symmetry of graphene is to stretch its lattice in one direction. -

Semiconductors Band Structure
Semiconductors Basic Properties Band Structure • Eg = energy gap • Silicon ~ 1.17 eV • Ge ~ 0.66 eV 1 Intrinsic Semiconductors • Pure Si, Ge are intrinsic semiconductors. • Some electrons elevated to conduction band by thermal energy. Fermi-Dirac Distribution • The probability that a particular energy state ε is filled is just the F-D distribution. • For intrinsic conductors at room temperature the chemical potential, µ, is approximately equal to the Fermi Energy, EF. • The Fermi Energy is in the middle of the band gap. 2 Conduction Electrons • If ε - EF >> kT then • If we measure ε from the top of the valence band and remember that EF lies in the middle of the band gap then Conduction Electrons • A full analysis taking into account the number of states per energy (density of states) gives an estimate for the fraction of electrons in the conduction band of 3 Electrons and Holes • When an electron in the valence band is excited into the conduction band it leaves behind a hole. Holes • The holes act like positive charge carriers in the valence band. Electric Field 4 Holes • In terms of energy level electrons tend to fall into lower energy states which means that the holes tends to rise to the top of the valence band. Photon Excitations • Photons can excite electrons into the conduction band as well as thermal fluctuations 5 Impurity Semiconductors • An impurity is introduced into a semiconductor (doping) to change its electronic properties. • n-type have impurities with one more valence electron than the semiconductor. • p-type have impurities with one fewer valence electron than the semiconductor. -

“Mechanical Universe and Beyond” Videos—CE Mungan, Spring 2001
Keywords in “Mechanical Universe and Beyond” Videos—C.E. Mungan, Spring 2001 This entire series can be viewed online at http://www.learner.org/resources/series42.html. Some of the titles below have been modified by me to better reflect their contents. In my opinion, tapes 21–22 are the best in the whole series! 1. Introduction to Classical Mechanics: Kepler, Galileo, Newton 2. Falling Bodies: s = gt2 / 2, ! = gt, a = g 3. Differentiation: introductory math 4. Inertia: Newton’s first law, Copernican solar system 5. Vectors: quaternions, unit vectors, dot and cross products 6. Newton’s Laws: Newton’s second law, momentum, Newton’s third law, monkey-gun demo 7. Integration: Newton vs. Leibniz, anti-derivatives 8. Gravity: planetary orbits, universal law of gravity, the Moon falls toward the Earth 9. UCM: Ptolemaic solar system, centripetal acceleration and force 10. Fundamental Forces: Cavendish experiment, Franklin, unified theory, viscosity, tandem accelerator 11. Gravity and E&M: fundamental constants, speed of light, Oersted experiment, Maxwell 12. Millikan Experiment: CRT, scientific method 13. Energy Conservation: work, gravitational PE, KE, mechanical energy, heat, Joule, microscopic forms of energy, useful available energy 14. PE: stability, conservation, position dependence, escape speed 15. Conservation of Linear Momentum: Descartes, generalized Newton’s second law, Earth- Moon system, linear accelerator 16. SHM: amplitude-independent period of pendulum, timekeeping, restoring force, connection to UCM, elastic PE 17. Resonance: Tacoma Narrows, music, breaking wineglass demo, earthquakes, Aeolian harp, vortex shedding 18. Waves: shock waves, speed of sound, coupled oscillators, wave properties, gravity waves, isothermal vs. adiabatic bulk modulus 19. Conservation of Angular Momentum: Kepler’s second law, vortices, torque, Brahe 20. -

Caltech News
Volume 16, No.7, December 1982 CALTECH NEWS pounds, became optional and were Three Caltech offered in the winter and spring. graduate programs But under this plan, there was an overlap in material that diluted the rank number one program's efficiency, blending per in nationwide survey sons in the same classrooms whose backgrounds varied widely. Some Caltech ranked number one - students took 3B and 3C before either alone or with other institutions proceeding on to 46A and 46B, - in a recent report that judged the which focused on organic systems, scholastic quality of graduate pro" while other students went directly grams in mathematics and science at into the organic program. the nation's major research Another matter to be addressed universities. stemmed from the fact that, across Caltech led the field in geoscience, the country, the lines between inor and shared top rankings with Har ganic and organic chemistry had ' vard in physics. The Institute was in become increasingly blurred. Explains a four-way tie for first in chemistry Professor of Chemistry Peter Der with Berkeley, Harvard, and MIT. van, "We use common analytical The report was the result of a equipment. We are both molecule two-year, $500,000 study published builders in our efforts to invent new under the sponsorship of four aca materials. We use common bonds for demic groups - the American Coun The Mead Laboratory is the setting for Chemistry 5, where Carlotta Paulsen uses a rotary probing how chemical bonds are evaporator to remove a solvent from a synthesized product. Paulsen is a junior majoring in made and broken." cil of Learned Societies, the American chemistry. -

Nuclear Magnetic Resonance and Its Application in Condensed Matter Physics
Nuclear Magnetic Resonance and Its Application in Condensed Matter Physics Kangbo Hao 1. Introduction Nuclear Magnetic Resonance (NMR) is a physics phenomenon first observed by Isidor Rabi in 1938. [1] Since then, the NMR spectroscopy has been applied in a wide range of areas such as physics, chemistry, and medical examination. In this paper, I want to briefly discuss about the theory of NMR spectroscopy and its recent application in condensed matter physics. 2. Principles of NMR NMR occurs when some certain nuclei are in a static magnetic field and another oscillation magnetic field. Assuming a nucleus has a spin angular momentum 퐼⃗ = ℏ푚퐼, then its magnetic moment 휇⃗ is 휇⃗ = 훾퐼⃗ (1) The 훾 here is the gyromagnetic ratio, which depends on the property of the nucleus. If we put such a nucleus in a static magnetic field 퐵⃗⃗0, then the magnetic moment of this nuclei will process about this magnetic field. Therefore we have, [2] [3] 푑퐼⃗ 1 푑휇⃗⃗⃗ 휏⃗ = 휇⃗ × 퐵⃗⃗ = = (2) 0 푑푥 훾 푑푥 From this semiclassical picture, we can easily derive that the precession frequency 휔0 (which is called the Larmor angular frequency) is 휔0 = 훾퐵0 (3) Then, if another small oscillating magnetic field is added to the plane perpendicular to 퐵⃗⃗0, then the total magnetic field is (Assuming 퐵⃗⃗0 is in 푧̂ direction) 퐵⃗⃗ = 퐵0푧̂ + 퐵1(cos(휔푡) 푥̂ + sin(휔푡) 푦̂) (4) If we choose a frame (푥̂′, 푦̂′, 푧̂′ = 푧̂) rotating with the oscillating magnetic field, then the effective magnetic field in this frame is 휔 퐵̂ = (퐵 − ) 푧̂ + 퐵 푥̂′ (5) 푒푓푓 0 훾 1 As a result, at 휔 = 훾퐵0, which is the resonant frequency, the 푧̂ component will vanish, and thus the spin angular momentum will precess about 퐵⃗⃗1 instead. -
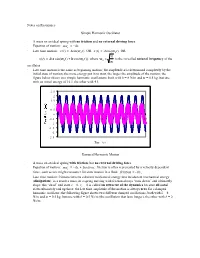
Notes on Resonance Simple Harmonic Oscillator • a Mass on An
Notes on Resonance Simple Harmonic Oscillator · A mass on an ideal spring with no friction and no external driving force · Equation of motion: max = - kx · Late time motion: x(t) = Asin(w0 t) OR x(t) = Acos(w0 t) OR k x(t) = A(a sin(w t) + b cos(w t)), where w = is the so-called natural frequency of the 0 0 0 m oscillator · Late time motion is the same as beginning motion; the amplitude A is determined completely by the initial state of motion; the more energy put in to start, the larger the amplitude of the motion; the figure below shows two simple harmonic oscillations, both with k = 8 N/m and m = 0.5 kg, but one with an initial energy of 16 J, the other with 4 J. 2.5 2 1.5 1 0.5 0 -0.5 0 5 10 15 20 -1 -1.5 -2 -2.5 Time (s) Damped Harmonic Motion · A mass on an ideal spring with friction, but no external driving force · Equation of motion: max = - kx + friction; friction is often represented by a velocity dependent force, such as one might encounter for slow motion in a fluid: friction = - bv x · Late time motion: friction converts coherent mechanical energy into incoherent mechanical energy (dissipation); as a result a mass on a spring moving with friction always “runs down” and ultimately stops; this “dead” end state x = 0, vx = 0 is called an attractor of the dynamics because all initial states ultimately end up there; the late time amplitude of the motion is always zero for a damped harmonic oscillator; the following figure shows two different damped oscillations, both with k = 8 N/m and m = 0.5 kg, but one with b = 0.5 Ns/m (the oscillation that lasts longer), the other with b = 2 Ns/m. -

Condensed Matter Physics Experiments List 1
Physics 431: Modern Physics Laboratory – Condensed Matter Physics Experiments The Oscilloscope and Function Generator Exercise. This ungraded exercise allows students to learn about oscilloscopes and function generators. Students measure digital and analog signals of different frequencies and amplitudes, explore how triggering works, and learn about the signal averaging and analysis features of digital scopes. They also explore the consequences of finite input impedance of the scope and and output impedance of the generator. List 1 Electron Charge and Boltzmann Constants from Johnson Noise and Shot Noise Mea- surements. Because electronic noise is an intrinsic characteristic of electronic components and circuits, it is related to fundamental constants and can be used to measure them. The Johnson (thermal) noise across a resistor is amplified and measured at both room temperature and liquid nitrogen temperature for a series of different resistances. The amplifier contribution to the mea- sured noise is subtracted out and the dependence of the noise voltage on the value of the resistance leads to the value of the Boltzmann constant kB. In shot noise, a series of different currents are passed through a vacuum diode and the RMS noise across a load resistor is measured at each current. Since the current is carried by electron-size charges, the shot noise measurements contain information about the magnitude of the elementary charge e. The experiment also introduces the concept of “noise figure” of an amplifier and gives students experience with a FFT signal analyzer. Hall Effect in Conductors and Semiconductors. The classical Hall effect is the basis of most sensors used in magnetic field measurements. -

AN826 Crystal Oscillator Basics and Crystal Selection for Rfpic™ And
AN826 Crystal Oscillator Basics and Crystal Selection for rfPICTM and PICmicro® Devices • What temperature stability is needed? Author: Steven Bible Microchip Technology Inc. • What temperature range will be required? • Which enclosure (holder) do you desire? INTRODUCTION • What load capacitance (CL) do you require? • What shunt capacitance (C ) do you require? Oscillators are an important component of radio fre- 0 quency (RF) and digital devices. Today, product design • Is pullability required? engineers often do not find themselves designing oscil- • What motional capacitance (C1) do you require? lators because the oscillator circuitry is provided on the • What Equivalent Series Resistance (ESR) is device. However, the circuitry is not complete. Selec- required? tion of the crystal and external capacitors have been • What drive level is required? left to the product design engineer. If the incorrect crys- To the uninitiated, these are overwhelming questions. tal and external capacitors are selected, it can lead to a What effect do these specifications have on the opera- product that does not operate properly, fails prema- tion of the oscillator? What do they mean? It becomes turely, or will not operate over the intended temperature apparent to the product design engineer that the only range. For product success it is important that the way to answer these questions is to understand how an designer understand how an oscillator operates in oscillator works. order to select the correct crystal. This Application Note will not make you into an oscilla- Selection of a crystal appears deceivingly simple. Take tor designer. It will only explain the operation of an for example the case of a microcontroller. -

Periodic Trends Lab CHM120 1The Periodic Table Is One of the Useful
Periodic Trends Lab CHM120 1The Periodic Table is one of the useful tools in chemistry. The table was developed around 1869 by Dimitri Mendeleev in Russia and Lothar Meyer in Germany. Both used the chemical and physical properties of the elements and their tables were very similar. In vertical groups of elements known as families we find elements that have the same number of valence electrons such as the Alkali Metals, the Alkaline Earth Metals, the Noble Gases, and the Halogens. 2Metals conduct electricity extremely well. Many solids, however, conduct electricity somewhat, but nowhere near as well as metals, which is why such materials are called semiconductors. Two examples of semiconductors are silicon and germanium, which lie immediately below carbon in the periodic table. Like carbon, each of these elements has four valence electrons, just the right number to satisfy the octet rule by forming single covalent bonds with four neighbors. Hence, silicon and germanium, as well as the gray form of tin, crystallize with the same infinite network of covalent bonds as diamond. 3The band gap is an intrinsic property of all solids. The following image should serve as good springboard into the discussion of band gaps. This is an atomic view of the bonding inside a solid (in this image, a metal). As we can see, each of the atoms has its own given number of energy levels, or the rings around the nuclei of each of the atoms. These energy levels are positions that electrons can occupy in an atom. In any solid, there are a vast number of atoms, and hence, a vast number of energy levels. -

Energy Bands in Crystals
Energy Bands in Crystals This chapter will apply quantum mechanics to a one dimensional, periodic lattice of potential wells which serves as an analogy to electrons interacting with the atoms of a crystal. We will show that as the number of wells becomes large, the allowed energy levels for the electron form nearly continuous energy bands separated by band gaps where no electron can be found. We thus have an interesting quantum system which exhibits many dual features of the quantum continuum and discrete spectrum. several tenths nm The energy band structure plays a crucial role in the theory of electron con- ductivity in the solid state and explains why materials can be classified as in- sulators, conductors and semiconductors. The energy band structure present in a semiconductor is a crucial ingredient in understanding how semiconductor devices work. Energy levels of “Molecules” By a “molecule” a quantum system consisting of a few periodic potential wells. We have already considered a two well molecular analogy in our discussions of the ammonia clock. 1 V -c sin(k(x-b)) V -c sin(k(x-b)) cosh βx sinhβ x V=0 V = 0 0 a b d 0 a b d k = 2 m E β= 2m(V-E) h h Recall for reasonably large hump potentials V , the two lowest lying states (a even ground state and odd first excited state) are very close in energy. As V →∞, both the ground and first excited state wave functions become completely isolated within a well with little wave function penetration into the classically forbidden central hump. -

Resonance Beyond Frequency-Matching
Resonance Beyond Frequency-Matching Zhenyu Wang (王振宇)1, Mingzhe Li (李明哲)1,2, & Ruifang Wang (王瑞方)1,2* 1 Department of Physics, Xiamen University, Xiamen 361005, China. 2 Institute of Theoretical Physics and Astrophysics, Xiamen University, Xiamen 361005, China. *Corresponding author. [email protected] Resonance, defined as the oscillation of a system when the temporal frequency of an external stimulus matches a natural frequency of the system, is important in both fundamental physics and applied disciplines. However, the spatial character of oscillation is not considered in the definition of resonance. In this work, we reveal the creation of spatial resonance when the stimulus matches the space pattern of a normal mode in an oscillating system. The complete resonance, which we call multidimensional resonance, is a combination of both the spatial and the conventionally defined (temporal) resonance and can be several orders of magnitude stronger than the temporal resonance alone. We further elucidate that the spin wave produced by multidimensional resonance drives considerably faster reversal of the vortex core in a magnetic nanodisk. Our findings provide insight into the nature of wave dynamics and open the door to novel applications. I. INTRODUCTION Resonance is a universal property of oscillation in both classical and quantum physics[1,2]. Resonance occurs at a wide range of scales, from subatomic particles[2,3] to astronomical objects[4]. A thorough understanding of resonance is therefore crucial for both fundamental research[4-8] and numerous related applications[9-12]. The simplest resonance system is composed of one oscillating element, for instance, a pendulum. Such a simple system features a single inherent resonance frequency.