MARCH 2017 I Vietnamese Journal of Primatology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Saving Habitats Saving Species Since 1989 Inside This
WLTnews ISSUE No. 61 SPRING 2019 Inside this issue: • £100 an acre: new opportunity in Jungle for Jaguars corridor • News in pictures: conservation stories from around the world • Field reports from Paraguay and Vietnam Saving habitats Saving species since 1989 worldlandtrust.org 1 New opportunity in the Jungle for Jaguars corridor Buy an acre of tropical forest for £100 COUNTRY: BELIZE LAND: 1,818 ACRES TARGET: £181,800 The success of our Jungle for Jaguars appeal has unlocked an opportunity to buy an additional piece of forest to add to the corridor. And the good news is that it is available for purchase at our Buy an Acre price of £100 per acre. The Jungle for Jaguars appeal in late 2018 was an incredible success. World Land Trust (WLT) supporters raised £600,000 for the purchase and protection of 8,154 acres in a vital wildlife corridor. This land has now been purchased by our partner the Corozal Sustainable Future Initiative (CSFI), who manage several of the protected areas in this corridor. The additional 1,818 acres would ensure safe passage for Jaguars on the eastern side of Shipstern Lagoon. Within this parcel of land live the Fireburn Community who have 187 acres of community lands. CSFI will be building trust and 1,818 acres for purchase engagement with them as well as helping Shipstern Lagoon to develop sustainable livelihoods. Protected areas Buy an Acre Donate online (worldlandtrust.org), Community land by phone (01986 874422) or by post (using the enclosed donation form). Roseate Spoonbill Shipstern Lagoon, with its mangrove islands and water bird colonies, is the largest inland lagoon in Belize. -

Drug War Monitor
WOLA Drug War Monitor FEBRUARY 2003 A WOLA BRIEFING SERIES The push for zero coca: Democratic A publication of WOLA’s “Drugs, Democracy and transition and counternarcotics policy in Peru Human Rights” project, which examines the By Isaías Rojas impact of drug traffick- ing and U.S. international “ e will not negotiate with this commission. We want face-to-face drug control policies on negotiations with (President Alejandro) Toledo. If he is not here by human rights and Wtomorrow morning, we will go to Lima to speak with him directly. We want democratization trends to talk with the ringmaster of the circus.” This was the response members of a throughout Latin government commission received from approximately three thousand coca-growing America and the farmers from the Apurímac and Ene River Valley (VRAE), who said that they had not Caribbean begun their difficult march to Lima, the capital, only to turn back midway, nor would they discuss their problems with a commission of second-rate authorities. That Thursday night, August 1, 2002, an official commission had traveled to the city of Ayacucho with the mission of stopping a peasant protest begun two days earlier. The farmers refused to recognize the negotiating capacity of the commission, despite its makeup of high-ranking members of the National Commission for Development and Life without Drugs (DEVIDA)1 and congressional legislators from the ruling party. The farmers said they wanted to speak with someone with decision-making authority, such as the prime minister. Their trump cards were the concurrent march and an indefinite general strike declared in the Apurímac River Valley. -

Downloaded from Brill.Com09/27/2021 09:14:05PM Via Free Access 218 Rode-Margono & Nekaris – Impact of Climate and Moonlight on Javan Slow Lorises
Contributions to Zoology, 83 (4) 217-225 (2014) Impact of climate and moonlight on a venomous mammal, the Javan slow loris (Nycticebus javanicus Geoffroy, 1812) Eva Johanna Rode-Margono1, K. Anne-Isola Nekaris1, 2 1 Oxford Brookes University, Gipsy Lane, Headington, Oxford OX3 0BP, UK 2 E-mail: [email protected] Keywords: activity, environmental factors, humidity, lunarphobia, moon, predation, temperature Abstract Introduction Predation pressure, food availability, and activity may be af- To secure maintenance, survival and reproduction, fected by level of moonlight and climatic conditions. While many animals adapt their behaviour to various factors, such nocturnal mammals reduce activity at high lunar illumination to avoid predators (lunarphobia), most visually-oriented nocturnal as climate, availability of resources, competition, preda- primates and birds increase activity in bright nights (lunarphilia) tion, luminosity, habitat fragmentation, and anthropo- to improve foraging efficiency. Similarly, weather conditions may genic disturbance (Kappeler and Erkert, 2003; Beier influence activity level and foraging ability. We examined the 2006; Donati and Borgognini-Tarli, 2006). According response of Javan slow lorises (Nycticebus javanicus Geoffroy, to optimal foraging theory, animal behaviour can be seen 1812) to moonlight and temperature. We radio-tracked 12 animals as a trade-off between the risk of being preyed upon in West Java, Indonesia, over 1.5 years, resulting in over 600 hours direct observations. We collected behavioural and environmen- and the fitness gained from foraging (Charnov, 1976). tal data including lunar illumination, number of human observ- Perceived predation risk assessed through indirect cues ers, and climatic factors, and 185 camera trap nights on potential that correlate with the probability of encountering a predators. -

Annual Review 2019 Registered Charity 1001291
ANNUAL REVIEW 2019 REGISTERED CHARITY 1001291 worldlandtrust.org A YEAR TO REMEMBER A FEW WORDS FROM Dr Gerard Bertrand Steve Backshall, MBE Hon President, WLT Patron For WLT so many years of the last three decades have been marked by One Wild Night 2019 outstanding progress in conservation. In December, Steve Backshall hosted This past year, however, was a magnificent evening at the Royal remarkable because the Trust recorded Geographical Society. its largest number and diversity of Playing to a packed house, Steve conservation projects throughout the welcomed on stage some of the UK’s world. The Trust truly is making a leading adventurers, explorers and difference for wildlife from Southeast sports personalities including his wife, Asia to Africa to the Americas. Helen Glover, Baroness Tanni Grey- the Río Zuñac appeal we launched on the Thompson, Dan Snow, Professor Ben evening to purchase cloud forest in It was also the year of successful Garrod, Phoebe Smith and Dwayne Ecuador has reached its fundraising transition from our incomparable Fields. Monty Don compered the target. Because of your support, WLT and founders John and Viv Burton to new evening and young environmental Fundación EcoMinga can expand the Trust leadership. The Trust is fortunate activist, Bella Lack spoke passionately reserve, securing it for the species that to have attracted an outstanding about the threats facing the planet and live here. conservationist, Dr. Jonathan Barnard as solutions within our grasp. We all know that we need to do the new Chief Executive to lead WLT. something to protect our planet. I believe The past three decades seem to have Steve says: the most practical step we can take is to flown by since John Burton and I “One Wild Night was filled with inspiring continue purchasing these remote hatched the plan to build a new stories of conservation and adventure in wildernesses, placing them in the hands organisation in the UK to support the wild world. -

In Situ Conservation
NEWSN°17/DECEMBER 2020 Editorial IN SITU CONSERVATION One effect from 2020 is for sure: Uncertainty. Forward planning is largely News from the Little Fireface First, our annual SLOW event was impossible. We are acting and reacting Project, Java, Indonesia celebrated world-wide, including along the current situation caused by the By Prof K.A.I. Nekaris, MA, PhD by project partners Kukang Rescue Covid-19 pandemic. All zoos are struggling Director of the Little Fireface Project Program Sumatra, EAST Vietnam, Love economically after (and still ongoing) Wildlife Thailand, NE India Primate temporary closures and restricted business. The Little Fireface Project team has Investments in development are postponed Centre India, and the Bangladesh Slow at least. Each budget must be reviewed. been busy! Despite COVID we have Loris Project, to name a few. The end In the last newsletter we mentioned not been able to keep up with our wild of the week resulted in a loris virtual to forget about the support of the in situ radio collared slow lorises, including conference, featuring speakers from conservation efforts. Some of these under welcoming many new babies into the the helm of the Prosimian TAG are crucial 11 loris range countries. Over 200 for the survival of species – and for a more family. The ‘cover photo’ you see here people registered, and via Facebook sustainable life for the people involved in is Smol – the daughter of Lupak – and Live, more than 6000 people watched rd some of the poorest countries in the world. is our first 3 generation birth! Having the event. -

Species of the Day: Pied Tamarin
© Gregory Guida Species of the Day: Pied Tamarin The Pied Tamarin or Brazilian Bare-faced Tamarin, Saguinus bicolor, is a small monkey endemic to the Brazilian Amazon, and is classified as ‘Endangered’ on the IUCN Red List of Threatened SpeciesTM. It occurs largely within and around the city of Manaus, in the heart of the Amazon basin, and has one of the smallest ranges of any primate. The expansion of Manaus has reduced much of the species’ habitat to mere fragments which Geographical range are disappearing rapidly, destroyed by people in search of land and by land-use planning www.iucnredlist.org that fails to take environmental needs into account. Tamarins migrating from one tiny patch of www.durrell.org forest to another are often electrocuted by power cables or are run over whilst crossing roads. Help Save Species www.arkive.org Translocation of these primates to safer patches of forest is now being implemented to help conserve this species. Seven potential conservation areas for Pied Tamarins have been identified. These areas require protection, as well as the creation of forest corridors to connect them, in order to secure the future of this species in the wild. On 18-29 October, officials will gather at the tenth meeting of the Conference of the Parties to the Convention on Biological Diversity (CBD COP10), in Nagoya, Japan, to agree how to tackle biodiversity loss. The production of the IUCN Red List of Threatened Species™ is made possible through the IUCN Red List Partnership: Species of the Day IUCN (including the Species Survival Commission), BirdLife is sponsored by International, Conservation International, NatureServe and Zoological Society of London.. -
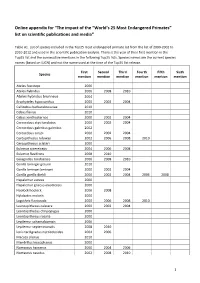
Online Appendix for “The Impact of the “World's 25 Most Endangered
Online appendix for “The impact of the “World’s 25 Most Endangered Primates” list on scientific publications and media” Table A1. List of species included in the Top25 most endangered primate list from the list of 2000-2002 to 2010-2012 and used in the scientific publication analysis. There is the year of their first mention in the Top25 list and the consecutive mentions in the following Top25 lists. Species names are the current species names (based on IUCN) and not the name used at the time of the Top25 list release. First Second Third Fourth Fifth Sixth Species mention mention mention mention mention mention Ateles fusciceps 2006 Ateles hybridus 2006 2008 2010 Ateles hybridus brunneus 2004 Brachyteles hypoxanthus 2000 2002 2004 Callicebus barbarabrownae 2010 Cebus flavius 2010 Cebus xanthosternos 2000 2002 2004 Cercocebus atys lunulatus 2000 2002 2004 Cercocebus galeritus galeritus 2002 Cercocebus sanjei 2000 2002 2004 Cercopithecus roloway 2002 2006 2008 2010 Cercopithecus sclateri 2000 Eulemur cinereiceps 2004 2006 2008 Eulemur flavifrons 2008 2010 Galagoides rondoensis 2006 2008 2010 Gorilla beringei graueri 2010 Gorilla beringei beringei 2000 2002 2004 Gorilla gorilla diehli 2000 2002 2004 2006 2008 Hapalemur aureus 2000 Hapalemur griseus alaotrensis 2000 Hoolock hoolock 2006 2008 Hylobates moloch 2000 Lagothrix flavicauda 2000 2006 2008 2010 Leontopithecus caissara 2000 2002 2004 Leontopithecus chrysopygus 2000 Leontopithecus rosalia 2000 Lepilemur sahamalazensis 2006 Lepilemur septentrionalis 2008 2010 Loris tardigradus nycticeboides -

Science Journals
SCIENCE ADVANCES | REVIEW PRIMATOLOGY 2017 © The Authors, some rights reserved; Impending extinction crisis of the world’s primates: exclusive licensee American Association Why primates matter for the Advancement of Science. Distributed 1 2 3 4 under a Creative Alejandro Estrada, * Paul A. Garber, * Anthony B. Rylands, Christian Roos, Commons Attribution 5 6 7 7 Eduardo Fernandez-Duque, Anthony Di Fiore, K. Anne-Isola Nekaris, Vincent Nijman, NonCommercial 8 9 10 10 Eckhard W. Heymann, Joanna E. Lambert, Francesco Rovero, Claudia Barelli, License 4.0 (CC BY-NC). Joanna M. Setchell,11 Thomas R. Gillespie,12 Russell A. Mittermeier,3 Luis Verde Arregoitia,13 Miguel de Guinea,7 Sidney Gouveia,14 Ricardo Dobrovolski,15 Sam Shanee,16,17 Noga Shanee,16,17 Sarah A. Boyle,18 Agustin Fuentes,19 Katherine C. MacKinnon,20 Katherine R. Amato,21 Andreas L. S. Meyer,22 Serge Wich,23,24 Robert W. Sussman,25 Ruliang Pan,26 27 28 Inza Kone, Baoguo Li Downloaded from Nonhuman primates, our closest biological relatives, play important roles in the livelihoods, cultures, and religions of many societies and offer unique insights into human evolution, biology, behavior, and the threat of emerging diseases. They are an essential component of tropical biodiversity, contributing to forest regeneration and ecosystem health. Current information shows the existence of 504 species in 79 genera distributed in the Neotropics, mainland Africa, Madagascar, and Asia. Alarmingly, ~60% of primate species are now threatened with extinction and ~75% have de- clining populations. This situation is the result of escalating anthropogenic pressures on primates and their habitats— mainly global and local market demands, leading to extensive habitat loss through the expansion of industrial agri- http://advances.sciencemag.org/ culture, large-scale cattle ranching, logging, oil and gas drilling, mining, dam building, and the construction of new road networks in primate range regions. -

Table 7: Species Changing IUCN Red List Status (2018-2019)
IUCN Red List version 2019-3: Table 7 Last Updated: 10 December 2019 Table 7: Species changing IUCN Red List Status (2018-2019) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2018 (IUCN Red List version 2018-2) and 2019 (IUCN Red List version 2019-3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2018) List (2019) change version Category -

Influence of External and Internal Environmental Factors on Intestinal Microbiota of Wild and Domestic Animals A
Influence of external and internal environmental factors on intestinal microbiota of wild and domestic of animals factors on intestinal microbiota Influence of external and internal environmental Influence of external and internal environmental factors on intestinal microbiota of wild and domestic animals A. Umanets Alexander Umanets Propositions 1. Intestinal microbiota and resistome composition of wild animals are mostly shaped by the animals’ diet and lifestyle. (this thesis) 2. When other environmental factors are controlled, genetics of the host lead to species- or breed specific microbiota patterns. (this thesis) 3. Identifying the response of microbial communities to factors that only have a minor contribution to overall microbiota variation faces the same problems as the discovery of exoplanets. 4. Observational studies in microbial ecology using cultivation- independent methods should be considered only as a guide for further investigations that employ controlled experimental conditions and mechanistic studies of cause-effect relationships. 5. Public fear of genetic engineering and artificial intelligence is not helped by insufficient public education and misleading images created through mass- and social media. 6. Principles of positive (Darwinian) and negative selection govern the repertoire of techniques used within martial arts. Propositions belonging to the thesis, entitled Influence of external and internal environmental factors on intestinal microbiota of wild and domestic animals Alexander Umanets Wageningen, 17 October -

Reverse the Red Australia Bushfire Crisis Introducing New WAZA
2020 02 NEWS Introducing New Reverse Australia WAZA CEO the Red Bushfire Crisis 1 WAZA Executive Office Staff Chief Executive Officer Martín Zordan [email protected] Chief Operating Officer Christina Morbin [email protected] Director of Communications Gavrielle Kirk-Cohen [email protected] Director of Membership Janet Ho [email protected] Animal Welfare Intern Paula Cerdán [email protected] Imprint WAZA Executive Office Contacts Postal Address WAZA Executive Office Editor: Carrer de Roger de Llúria 2, 2-2 Gavrielle Kirk-Cohen 08010 Barcelona Spain Reviewer: Phone +34 936638811 Paula Cerdán Email [email protected] Website www.waza.org Proofreader: Facebook @officialWAZA Laurie Clinton Instagram @wazaglobal Linkedin @World Association Zoos & Aquariums Layout and design: @waza Smith&Brown.eu Twitter This edition of WAZA News is WAZA Membership also available at: www.waza.org WAZA members as of 14 April 2020 Printed on FSC-certified paper Affiliates 10 Associations 24 Corporates 18 Institutions 282 Future Events Cover Photo: A koala receives treatment 2020: Virtual Conference, October for burns sustained in the Australian 2021: Moscow Zoo, Moscow, Russia bushfires. Credit:© Zoos Victoria 2022: Loro Parque, Tenerife, Spain 2 President’s Letter Prof Theo B. Pagel President of WAZA Dear colleagues, The year so far has been incredibly challenging. We find ourselves in a surreal situation of continuing to operate institutions that are now closed to the public as a result of COVID-19. This places an enormous personal and organisational strain but our future potential is undiminished and we learn as we progress. It is now clear to everyone that we live in one global and interconnected world and society. -

Controlled Alien Species -Common Name
Controlled Alien Species –Common Name List of Controlled Alien Species Amphibians by Common Name -3 species- (Updated December 2009) Frogs Common Name Family Genus Species Poison Dart Frog, Black-Legged Dendrobatidae Phyllobates bicolor Poison Dart Frog, Golden Dendrobatidae Phyllobates terribilis Poison Dart Frog, Kokoe Dendrobatidae Phyllobates aurotaenia Page 1 of 50 Controlled Alien Species –Common Name List of Controlled Alien Species Birds by Common Name -3 species- (Updated December 2009) Birds Common Name Family Genus Species Cassowary, Dwarf Cassuariidae Casuarius bennetti Cassowary, Northern Cassuariidae Casuarius unappendiculatus Cassowary, Southern Cassuariidae Casuarius casuarius Page 2 of 50 Controlled Alien Species –Common Name List of Controlled Alien Species Mammals by Common Name -437 species- (Updated March 2010) Common Name Family Genus Species Artiodactyla (Even-toed Ungulates) Bovines Buffalo, African Bovidae Syncerus caffer Gaur Bovidae Bos frontalis Girrafe Giraffe Giraffidae Giraffa camelopardalis Hippopotami Hippopotamus Hippopotamidae Hippopotamus amphibious Hippopotamus, Madagascan Pygmy Hippopotamidae Hexaprotodon liberiensis Carnivora Canidae (Dog-like) Coyote, Jackals & Wolves Coyote (not native to BC) Canidae Canis latrans Dingo Canidae Canis lupus Jackal, Black-Backed Canidae Canis mesomelas Jackal, Golden Canidae Canis aureus Jackal Side-Striped Canidae Canis adustus Wolf, Gray (not native to BC) Canidae Canis lupus Wolf, Maned Canidae Chrysocyon rachyurus Wolf, Red Canidae Canis rufus Wolf, Ethiopian