ED 042 219 DOCUMENT RESUME CG 005 800 the Development of A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exposures Associated with Clandestine Methamphetamine Drug Laboratories in Australia
Rev Environ Health 2016; 31(3): 329–352 Jackie Wright*, John Edwards and Stewart Walker Exposures associated with clandestine methamphetamine drug laboratories in Australia DOI 10.1515/reveh-2016-0017 Received April 20, 2016; accepted June 7, 2016; previously published Introduction online July 18, 2016 Illicit drugs such as amphetamine-type stimulants (ATS) Abstract: The clandestine manufacture of methamphet- (1) are manufactured in Australia within clandestine amine in residential homes may represent significant laboratories that range from crude, makeshift operations hazards and exposures not only to those involved in the using simple processes to sophisticated operations. These manufacture of the drugs but also to others living in the laboratories use a range of chemical precursors to manu- home (including children), neighbours and first respond- facture or “cook” ATS that include methylamphetamine, ers to the premises. These hazards are associated with more commonly referred to as methamphetamine (“ice”) the nature and improper storage and use of precursor and 3,4-methylenedioxymethamphetamine (MDMA or chemicals, intermediate chemicals and wastes, gases and “ecstasy”). In Australia the primary ATS manufactured methamphetamine residues generated during manufac- in clandestine drug laboratories is methamphetamine ture and the drugs themselves. Many of these compounds (2), which is the primary focus of this review. Clandes- are persistent and result in exposures inside a home not tine laboratories are commonly located within residential only during manufacture but after the laboratory has been homes, units, hotel rooms, backyard sheds and cars, with seized or removed. Hence new occupants of buildings for- increasing numbers detected in Australia each year (744 merly used to manufacture methamphetamine may be laboratories detected in 2013–2014) (2). -

Nitrosamines EMEA-H-A5(3)-1490
25 June 2020 EMA/369136/2020 Committee for Medicinal Products for Human Use (CHMP) Assessment report Procedure under Article 5(3) of Regulation EC (No) 726/2004 Nitrosamine impurities in human medicinal products Procedure number: EMEA/H/A-5(3)/1490 Note: Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ...................................................................................... 2 1. Information on the procedure ............................................................... 7 2. Scientific discussion .............................................................................. 7 2.1. Introduction......................................................................................................... 7 2.2. Quality and safety aspects ..................................................................................... 7 2.2.1. Root causes for presence of N-nitrosamines in medicinal products and measures to mitigate them............................................................................................................. 8 2.2.2. Presence and formation of N-nitrosamines -

Recommended Methods for the Identification and Analysis Of
Vienna International Centre, P.O. Box 500, 1400 Vienna, Austria Tel: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org RECOMMENDED METHODS FOR THE IDENTIFICATION AND ANALYSIS OF AMPHETAMINE, METHAMPHETAMINE AND THEIR RING-SUBSTITUTED ANALOGUES IN SEIZED MATERIALS (revised and updated) MANUAL FOR USE BY NATIONAL DRUG TESTING LABORATORIES Laboratory and Scientific Section United Nations Office on Drugs and Crime Vienna RECOMMENDED METHODS FOR THE IDENTIFICATION AND ANALYSIS OF AMPHETAMINE, METHAMPHETAMINE AND THEIR RING-SUBSTITUTED ANALOGUES IN SEIZED MATERIALS (revised and updated) MANUAL FOR USE BY NATIONAL DRUG TESTING LABORATORIES UNITED NATIONS New York, 2006 Note Mention of company names and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. ST/NAR/34 UNITED NATIONS PUBLICATION Sales No. E.06.XI.1 ISBN 92-1-148208-9 Acknowledgements UNODC’s Laboratory and Scientific Section wishes to express its thanks to the experts who participated in the Consultative Meeting on “The Review of Methods for the Identification and Analysis of Amphetamine-type Stimulants (ATS) and Their Ring-substituted Analogues in Seized Material” for their contribution to the contents of this manual. Ms. Rosa Alis Rodríguez, Laboratorio de Drogas y Sanidad de Baleares, Palma de Mallorca, Spain Dr. Hans Bergkvist, SKL—National Laboratory of Forensic Science, Linköping, Sweden Ms. Warank Boonchuay, Division of Narcotics Analysis, Department of Medical Sciences, Ministry of Public Health, Nonthaburi, Thailand Dr. Rainer Dahlenburg, Bundeskriminalamt/KT34, Wiesbaden, Germany Mr. Adrian V. Kemmenoe, The Forensic Science Service, Birmingham Laboratory, Birmingham, United Kingdom Dr. Tohru Kishi, National Research Institute of Police Science, Chiba, Japan Dr. -

Page 498 TITLE 21—FOOD AND
§ 695 TITLE 21—FOOD AND DRUGS Page 498 pealed the permanent appropriation under the title Sec. ‘‘Meat inspection, Bureau of Animal Industry (fiscal 822. Persons required to register. year) (3–114)’’ effective July 1, 1935, provided that such 823. Registration requirements. portions of any Acts as make permanent appropriations 824. Denial, revocation, or suspension of registra- to be expended under such account are amended so as tion. to authorize, in lieu thereof, annual appropriations 825. Labeling and packaging. from the general fund of the Treasury in identical 826. Production quotas for controlled substances. terms and in such amounts as now provided by the laws 827. Records and reports of registrants. providing such permanent appropriations, and author- 828. Order forms. ized, in addition thereto, the appropriation of ‘‘such 829. Prescriptions. other sums as may be necessary in the enforcement of 830. Regulation of listed chemicals and certain the meat inspection laws.’’ In the original, the par- machines. enthetical ‘‘(U.S.C., title 21, secs. 71 to 96, inclusive)’’ 831. Additional requirements relating to online followed the phrase ‘‘meat inspection laws’’. The ‘‘meat pharmacies and telemedicine. inspection laws’’ are classified generally to this chap- ter. PART D—OFFENSES AND PENALTIES Section was not enacted as part of the Federal Meat 841. Prohibited acts A. Inspection Act which is classified to subchapters I to 842. Prohibited acts B. IV–A of this chapter. 843. Prohibited acts C. Section was formerly classified to section 95 of this 844. Penalties for simple possession. title. 844a. Civil penalty for possession of small amounts § 695. Payment of cost of meat-inspection service; of certain controlled substances. -

Development of Poly Unsaturated Fatty Acid Derivatives of Aspirin for Inhibition of Platelet Function S
Supplemental material to this article can be found at: http://jpet.aspetjournals.org/content/suppl/2016/08/03/jpet.116.234781.DC1 1521-0103/359/1/134–141$25.00 http://dx.doi.org/10.1124/jpet.116.234781 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 359:134–141, October 2016 Copyright ª 2016 by The American Society for Pharmacology and Experimental Therapeutics Development of Poly Unsaturated Fatty Acid Derivatives of Aspirin for Inhibition of Platelet Function s Jahnabi Roy, Reheman Adili, Richard Kulmacz, Michael Holinstat, and Aditi Das Department of Chemistry (J.R.), Division of Nutritional Sciences, Departments of Comparative Biosciences, Biochemistry, and Bioengineering, Center for Biophysics and Quantitative Biology, Beckman Institute for Advanced Science (A.D.), University of Illinois at Urbana-Champaign, Urbana, Illinois; Division of Cardiovascular Medicine (M.H.), Department of Pharmacology (R.A., M.H.), University of Michigan Medical School, Ann Arbor, Michigan; and Department of Internal Medicine, Texas Health Science Center, McGovern Medical School, Houston, Texas (R.K.) Received April 27, 2016; accepted August 1, 2016 Downloaded from ABSTRACT The inhibition of platelet aggregation is key to preventing particular, the aspirin-DHA anhydride displayed similar effective- conditions such as myocardial infarction and ischemic stroke. ness to aspirin. Platelet aggregation studies conducted in the Aspirin is the most widely used drug to inhibit platelet aggre- presence of various platelet agonists indicated that the aspirin-lipid gation. Aspirin absorption can be improved further to increase conjugates act through inhibition of the cyclooxygenase (COX)– jpet.aspetjournals.org its permeability across biologic membranes via esterification thromboxane synthase (TXAS) pathway. -

Enhancing Transdermal Delivery of Opioid Antagonists and Agonists Using Codrugs Linked to Bupropion Or Hydroxybupropion Audra L
University of Kentucky UKnowledge Pharmaceutical Sciences Faculty Patents Pharmaceutical Sciences 2-18-2014 Enhancing Transdermal Delivery of Opioid Antagonists and Agonists Using Codrugs Linked To Bupropion or Hydroxybupropion Audra L. Stinchcomb University of Kentucky Peter A. Crooks University of Kentucky, [email protected] Mohamad O. Hamad University of Kentucky, [email protected] Paul K. Kiptoo University of Kentucky Click here to let us know how access to this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/ps_patents Part of the Pharmacy and Pharmaceutical Sciences Commons Recommended Citation Stinchcomb, Audra L.; Crooks, Peter A.; Hamad, Mohamad O.; and Kiptoo, Paul K., "Enhancing Transdermal Delivery of Opioid Antagonists and Agonists Using Codrugs Linked To Bupropion or Hydroxybupropion" (2014). Pharmaceutical Sciences Faculty Patents. 27. https://uknowledge.uky.edu/ps_patents/27 This Patent is brought to you for free and open access by the Pharmaceutical Sciences at UKnowledge. It has been accepted for inclusion in Pharmaceutical Sciences Faculty Patents by an authorized administrator of UKnowledge. For more information, please contact [email protected]. US008653271B2 (12) United States Patent (10) Patent N0.: US 8,653,271 B2 Stinchcomb et a]. (45) Date of Patent: Feb. 18, 2014 (54) ENHANCING TRANSDERMAL DELIVERY (58) Field of Classi?cation Search OF OPIOID ANTAGONISTS AND AGONISTS USPC ............................... .. 546/45, 44, 46; 514/282 USING CODRUGS LINKED TO BUPROPION See application ?le for complete search history. OR HYDROXYBUPROPION (56) References Cited (75) Inventors: Audra L. Stinchcomb, Lexington, KY (US); Peter A. Crooks, Nicholasville, U.S. PATENT DOCUMENTS KY (US); Mohamed O. Hamad, Lexington, KY (US); Paul K. -

Detection and Characterization of Pyromarkers of Smoked Drugs of Abuse
Graduate Theses, Dissertations, and Problem Reports 2012 Detection and characterization of pyromarkers of smoked drugs of abuse Rona Kiyomi Nishikawa West Virginia University Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Nishikawa, Rona Kiyomi, "Detection and characterization of pyromarkers of smoked drugs of abuse" (2012). Graduate Theses, Dissertations, and Problem Reports. 4904. https://researchrepository.wvu.edu/etd/4904 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. DETECTION AND CHARACTERIZATION OF PYROMARKERS OF SMOKED DRUGS OF ABUSE Rona Kiyomi Nishikawa Dissertation submitted to the Eberly College of Arts and Sciences at West Virginia University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Chemistry Committee of Advisors: Suzanne C. Bell, Ph.D., Chair R. Lloyd Carroll, Ph.D. Patrick S. Callery, Ph.D. Harry O. Finklea, Ph.D. Keith B. Morris, Ph.D. C. Eugene Bennett Department of Chemistry Morgantown, West Virginia 2012 Keywords: Fentanyl, Heroin, Pyrolysis, Pyromarkers, GC/MS Copyright © 2012 Rona Kiyomi Nishikawa 1 Abstract DETECTION AND CHARACTERIZATION OF PYROMARKERS OF SMOKED DRUGS OF ABUSE Rona Kiyomi Nishikawa Smoking or inhalation has increased in popularity as the choice route of administration of drugs of abuse amongst drug abusers. -

Synthesis and Characterization of TPGS–Gemcitabine Prodrug
RSC Advances View Article Online PAPER View Journal | View Issue Synthesis and characterization of TPGS– gemcitabine prodrug micelles for pancreatic Cite this: RSC Adv.,2016,6,60126 cancer therapy† Vaibhav Khare,ab Wejdan Al. Sakarchi,a Prem N. Gupta,b Anthony D. M. Curtisa and Clare Hoskins*a The therapeutic potential of a nucleoside analog, gemcitabine, is severely compromised due to its rapid clearance from systemic circulation by enzymatic degradation into an inactive metabolite. In the present investigation, micelles based on polymer–drug conjugate were developed for gemcitabine and investigated for their potential to improve cancer chemotherapy. The tocopherol poly(ethylene glycol) succinate 1000 (TPGS)–gemcitabine prodrug was synthesized via an amide linkage and characterised by analytical methods, including FT-IR, 1H NMR, and MALDI-TOF. The micellar formulation of TPGS– gemcitabine prodrug was developed by a self-assembly technique and evaluated for various Creative Commons Attribution-NonCommercial 3.0 Unported Licence. physicochemical parameters including particle size, polydispersity, morphology, critical micelle concentration and release profile. It was observed that gemcitabine present in TPGS–gemcitabine micelles was resistant to deamination by crude cytidine deaminase. The improved cytotoxicity of the micellar formulation was observed using TPGS–gemcitabine micelles against pancreatic cancer cells. Received 11th April 2016 Further, it was found that, unlike native gemcitabine, nucleoside transporters were not required for Accepted 14th June 2016 TPGS–Gem micelles to demonstrate their anticancer potential. These findings revealed that TPGS– DOI: 10.1039/c6ra09347g gemcitabine micelles may serve as a promising platform for gemcitabine in order to improve its www.rsc.org/advances anticancer efficacy. -

Claire Cole, Lisa Jones, Jim Mcveigh, Andrew Kicman, Qutub Syed & Mark A
Claire Cole, Lisa Jones, Jim McVeigh, Andrew Kicman, Qutub Syed & Mark A. Bellis Acknowledgements The authors would like to thank the following for their contribution to the literature searching, design, proofing, editing and distribution of this document: Carol Chadwick. Sian Connolly, Layla English, Michael Evans-Brown, Dave Seddon, Olivia Wooding, Lee Tisdall, Ellie McCoy and Gemma Parry of the Centre for Public Health. We would also like to thank the International Harm Reduction Association (IHRA) for their support with launching this report at their 2010 conference. About the Authors Claire Cole and Lisa Jones are Senior Researchers at the Centre for Public Health, Liverpool John Moores University. Jim McVeigh is the Deputy Director and Reader in Substance Use Epidemiology at the Centre for Public Health. Professor Andrew Kicman is Head of Research and Development in the Department of Forensic Science and Drug Monitoring, The Drug Control Centre, at Kings College London. Professor Qutub Syed is the Regional Director for Health Protection Agency in the North West Region. Professor Mark A. Bellis is the Director of the Centre for Public Health. 1 Contents Executive Summary 4 1. Introduction 10 2. Methodology 13 3. Heroin 17 4. Cocaine and Crack Cocaine 25 5. Amphetamine and Methamphetamine 30 6. Ecstasy 33 7. Cannabis 36 8. Other Illicit Drugs 38 9. Public Health Response and Harm Reduction Messages 40 10. Summary 45 References 47 Appendix 1 - Glossary 55 List of Tables Table 1: Summary of drug adulteration evidence 5 Table 2: Summary -

Prodrug Derivatives of Carboxylic Acid Drugs
Patentamt JEuropaischesEuropean Patent Office Office europeen des brevets © Publication number: 0 278 977 B1 © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification : © Int. CI.5 : C07C 233/01, A61 K 31/21 06.05.92 Bulletin 92/19 © Application number : 87905946.7 @ Date of filing : 25.08.87 © International application number : PCT/DK87/00104 @ International publication number : WO 88/01615 10.03.88 Gazette 88/06 (54) PRODRUG DERIVATIVES OF CARBOXYLIC ACID DRUGS. The file contains technical information (56) References cited : submitted after the application was filed and US-A- 4 588 525 not included in this specification US-A- 4 678 806 CHEMICAL ABSTRACTS, vol. 106, 1987, ab- stract no. 781 87t, 16 March 1987; H. © 26.08.86 DK 4066/86 Priority : BUNDGAARD et al, Esters of N,N-disub- stituted2-hydroxyacetamides as a novel high- © Date of publication of application : ly biolabile prodrug type for carboxylic acid 24.08.88 Bulletin 88/34 agents'' © Publication of the grant of the patent : © Proprietor : Bundgaard, Hans 06.05.92 Bulletin 92/19 36, Tjornevej DK-2970 Horsholm (DK) Niels Mork States Proprietor : NIELSEN, © Designated Contracting : st.th. AT BE CH DE FR GB IT LI LU NL SE Cumberlandsgade 15, DK-2300 Kobenhavn S (DK) References cited 56 : Inventor Hans EP-A- 0 073 397 © : Bundgaard, EP-A- 077 720 36, Tjornevej DK-2970 Horsholm EP-A- 106 541 (DK) Inventor : NIELSEN, Niels Mork EP-A- 201 829 st.th. EP-A- 224 178 Cumberlandsgade 15, DK-2300 Kobenhavn S EP-A- 227 355 (DK) EP-A- 237 051 WO-A-86/00066 © Representative : Andersen, Henrik Rastrup et CH-A- 513 815 al US-A- 4 206 220 c/o Plougmann & Vingtoft Sankt Annae Plads US-A- 4 235 887 11 P.O. -
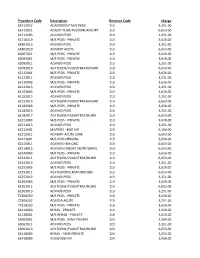
Procedure Code
Procedure Code Description Revenue Code Charge 61413012 ADJUSTMENT M/S PEDS 113 3,251.00 61413021 ADJUST TELM/PULM/NURO/ORT 113 6,653.00 61713103 ADJ M/S PEDS 113 3,251.00 61716119 M/S PEDS - PRIVATE 113 3,626.00 61801013 ADJ M/S PEDS 113 3,251.00 61802019 ADJ BMT ACUTE 113 6,653.00 62067021 M/S PEDS - PRIVATE 113 3,626.00 62093001 M/S PEDS - PRIVATE 113 3,626.00 62093011 ADJ M/S PEDS 113 3,251.00 62093013 ADJ TELEM/PULM/TRACH/NURO 113 6,653.00 62123001 M/S PEDS - PRIVATE 113 3,626.00 62123011 ADJ M/S PEDS 113 3,251.00 62133003 M/S PEDS - PRIVATE 113 3,626.00 62133013 ADJ M/S PEDS 113 3,251.00 62153003 M/S PEDS - PRIVATE 113 3,626.00 62153013 ADJ M/S PEDS 113 3,251.00 62153015 ADJ TELEM/PULM/TRACH/NURO 113 6,653.00 62183003 M/S PEDS - PRIVATE 113 3,626.00 62183013 ADJ M/S PEDS 113 3,251.00 62183017 ADJ TELEM/PULM/TRAC/NURO 113 6,653.00 62211003 M/S PEDS - PRIVATE 113 3,626.00 62211015 ADJ M/S PEDS 113 3,251.00 62212001 MS/PEDS - BMT HIP 113 6,160.00 62212011 ADJ BMT-ACUTE CARE 113 6,653.00 62213001 M/S PEDS HEMONC 113 3,626.00 62213011 ADJ M/S HEM ONC 113 6,653.00 62214015 ADJ M/S CARDIAC MONITORNG 113 6,653.00 62243003 M/S PEDS - PRIVATE 113 3,626.00 62243011 ADJ TELEM/PULM/TRAC/NURO 113 6,653.00 62243013 ADJ M/S PEDS 113 3,251.00 62253003 M/S PEDS - PRIVATE 113 3,626.00 62253011 ADJ TELM/PULM/NURO/OBS 113 6,653.00 62253013 ADJ M/S PEDS 113 3,251.00 62263003 M/S PEDS - PRIVATE 113 3,626.00 62263011 ADJ TELEM/PULM/TRAC/NURO 113 6,653.00 62263013 ADJ M/S PEDS 113 3,251.00 72309250 M/S PEDS - PRIVATE 113 3,626.00 72309252 ADJ EDA ACUTE 113 -

Polypharmacy Side Effect Prediction with Graph Convolutional Neural Network Based on Heterogeneous Structural and Biological Data
DEGREE PROJECT IN MATHEMATICS, SECOND CYCLE, 30 CREDITS STOCKHOLM, SWEDEN 2020 Polypharmacy Side Effect Prediction with Graph Convolutional Neural Network based on Heterogeneous Structural and Biological Data JUAN SEBASTIAN DIAZ BOADA KTH ROYAL INSTITUTE OF TECHNOLOGY SCHOOL OF ENGINEERING SCIENCES Polypharmacy Side Effect Prediction with Graph Convolutional Neural Network based on Heterogeneous Structural and Biological Data JUAN SEBASTIAN DIAZ BOADA Degree Projects in Scientific Computing (30 ECTS credits) Master’s Programme in Computer Simulations for Science and Engineering KTH Royal Institute of Technology year 2020 Supervisor at KI Algorithmic Dynamics Lab, Center for Molecular Medicine: Narsis A. Kiani Supervisor at KTH: Michael Hanke Examiner at KTH: Michael Hanke TRITA-SCI-GRU 2020:390 MAT-E 2020:097 Royal Institute of Technology School of Engineering Sciences KTH SCI SE-100 44 Stockholm, Sweden URL: www.kth.se/sci iii Acknowledgements This thesis and its experiments were performed in the Algorithmic Dynamics Lab of the Center for Molecular Medicine. Special thanks to Amir Amanzadi for creating the affinity score dataset, Jesper Tegnér for his comments analyz- ing results and Linus Johnson for his help with the Swedish translation. v Abstract The prediction of polypharmacy side effects is crucial to reduce the mortal- ity and morbidity of patients suffering from complex diseases. However, its experimental prediction is unfeasible due to the many possible drug combi- nations, leaving in silico tools as the most promising way of addressing this problem. This thesis improves the performance and robustness of a state-of- the-art graph convolutional network designed to predict polypharmacy side effects, by feeding it with complexity properties of the drug-protein network.