01 Nematodes.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nematoda: Enoplida) from the Tropics
Opusc. Zool. Budapest, (2006) 2008, 37: 3–9 Two new and a known species of the family Tripylidae (Nematoda: Enoplida) from the tropics I. ANDRÁSSY1 Abstract. Two new species of the genus Tripyla and Tripylella, respectively, as well as a known but rare species of the genus Tripylina are described and illustrated. Tripyla pulchella sp. n. from Papua New Guinea belongs to the smallest members of the genus, and is characterized by the narrowed anterior end, the well developed cephalic setae, the sclerotized vulval lips, the medium long, conical tail and the long terminal spinneret. Tripylella iucunda sp. n. from La Réunion is the shortest species within the genus possessing short cephalic setae, discoidal cardia and dorsally abruptly narrowed tail. Tripylina stramenti was found in São Tomé and is redescribed for the first time after the original description. wo new and a known but rare nematode spe- a = 19–21; b = 4.3–4.7; c = 6.1–6.4; c’ = 4.7–5.2; Tcies of the family Tripylidae found in tropical V = 54–59 %. regions of Earth are presented and described. They belong to the genera Tripyla, Tripylella and General description. A small and stout nema- Tripylina, respectively. tode, irregularly bent or twisted after fixation; bo- dy 36–42 µm wide at mid-region. Cuticle 1.5–2.0 The specimens of the new species were col- µm thick; finely annulated, annules 1.0–1.5 µm lected in Papua New Guinea and La Réunion, re- wide; annulations most conspicuous at vulval re- spectively, those of the known species in São gion and tail. -

Zootaxa, New Zealand Species of the Genus Tripyla Bastian, 1865
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/228485207 New Zealand species of the genus Tripyla Bastian, 1865 (Nematoda: Triplonchida: Tripylidae). I: A new species, a new record and key to long-tailed species Article · December 2009 CITATIONS READS 4 257 2 authors, including: Zeng Qi Zhao Manaaki Whenua - Landcare Research 69 PUBLICATIONS 454 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: An index to new genera and species of Nematoda in Zootaxa from 2007 to 2012 View project All content following this page was uploaded by Zeng Qi Zhao on 12 November 2015. The user has requested enhancement of the downloaded file. Zootaxa 2291: 35–50 (2009) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2009 · Magnolia Press ISSN 1175-5334 (online edition) New Zealand species of the genus Tripyla Bastian, 1865 (Nematoda:Triplonchida: Tripylidae). I : A new species, a new record and key to long-tailed species ZENG QI ZHAO Landcare Research, Private Bag 92170, Auckland Mail Centre, Auckland 1142, New Zealand Email: [email protected] Abstract This paper describes two species of the genus of Tripyla from New Zealand and also provides a key to species based on the morphology of females in eight long-tailed (c < 5) species in the genus of Tripyla. Tripyla bioblitz sp. nov. is characterized by its more anterior vulva position (V = 43.7–45.4%), relatively short body length (1150–1410 µm) and long tail (c = 4.0–4.4) in the group. -
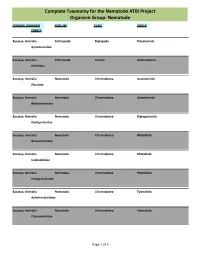
Complete Taxonomy for the Nematode ATBI Project Organism Group: Nematode
Complete Taxonomy for the Nematode ATBI Project Organism Group: Nematode DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Animalia Arthropoda Diplopoda Polydesmida Xystodesmidae Eucarya, Animalia Arthropoda Insecta Hymenoptera Halictidae Eucarya, Animalia Nematoda Chromadorea Araeolaimida Plectidae Eucarya, Animalia Nematoda Chromadorea Araeolaimida Rhabdolaimidae Eucarya, Animalia Nematoda Chromadorea Diplogasterida Diplogasteridae Eucarya, Animalia Nematoda Chromadorea Rhabditida Bunonematidae Eucarya, Animalia Nematoda Chromadorea Rhabditida Cephalobidae Eucarya, Animalia Nematoda Chromadorea Rhabditida Panagrolaimidae Eucarya, Animalia Nematoda Chromadorea Tylenchida Aphelenchoididae Eucarya, Animalia Nematoda Chromadorea Tylenchida Criconematidae Page 1 of 4 Complete Taxonomy for the Nematode ATBI Project Organism Group: Nematode DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Animalia Nematoda Chromadorea Tylenchida Hoplolaimidae Eucarya, Animalia Nematoda Enoplea Dorylaimida Actinolaimidae Eucarya, Animalia Nematoda Enoplea Dorylaimida Aporcelaimidae Eucarya, Animalia Nematoda Enoplea Dorylaimida Dorylaimidae Eucarya, Animalia Nematoda Enoplea Dorylaimida Longidoridae Eucarya, Animalia Nematoda Enoplea Dorylaimida Nygolaimidae Eucarya, Animalia Nematoda Enoplea Dorylaimida Qudsianematidae Eucarya, Animalia Nematoda Enoplea Enoplida Alaimidae Eucarya, Animalia Nematoda Enoplea Enoplida Ironidae Eucarya, Animalia Nematoda Enoplea Enoplida Prismatolaimidae Page 2 of 4 Complete Taxonomy for the Nematode ATBI Project Organism Group: -

Taxonomy Assignment Approach Determines the Efficiency of Identification of Metabarcodes in Marine Nematodes
Taxonomy assignment approach determines the efficiency of identification of metabarcodes in marine nematodes Oleksandr Holovachov1, Quiterie Haenel2, Sarah J. Bourlat3 and Ulf Jondelius1 1Department of Zoology, Swedish Museum of Natural History, Stockholm, Sweden 2Zoological Institute, University of Basel, Switzerland 3Department of Marine Sciences, University of Gothenburg, Sweden Abstract: Precision and reliability of barcode-based biodiversity assessment can be affected at several steps during acquisition and analysis of the data. Identification of barcodes is one of the crucial steps in the process and can be accomplished using several different approaches, namely, alignment-based, probabilistic, tree-based and phylogeny-based. Number of identified sequences in the reference databases affects the precision of identification. This paper compares the identification of marine nematode barcodes using alignment-based, tree-based and phylogeny-based approaches. Because the nematode reference dataset is limited in its taxonomic scope, barcodes can only be assigned to higher taxonomic categories, families. Phylogeny-based approach using Evolutionary Placement Algorithm provided the largest number of positively assigned metabarcodes and was least affected by erroneous sequences and limitations of reference data, comparing to alignment- based and tree-based approaches. Key words: biodiversity, identification, barcode, nematodes, meiobenthos. 1 1. Introduction Metabarcoding studies based on high throughput sequencing of amplicons from marine samples have reshaped our understanding of the biodiversity of marine microscopic eukaryotes, revealing a much higher diversity than previously known [1]. Early metabarcoding of the slightly larger sediment-dwelling meiofauna have mainly focused on scoring relative diversity of taxonomic groups [1-3]. The next step in metabarcoding: identification of species, is limited by the available reference database, which is sparse for most marine taxa, and by the matching algorithms. -

A Taxonomic Hierarchy and Checklist of the Genera and Higher Taxa of Marine Nematodes
A Taxonomic Hierarchy and Checklist of the Genera and Higher Taxa of Marine Nematodes w. D. HOPE and D. G. MURPHY SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 137 SERIAL PUBLICATIONS OF THE SMITHSONIAN INSTITUTION The emphasis upon publications as a means of diffusing knowledge was expressed by the first Secretary of the Smithsonian Institution. In his formal plan for the Insti- tution, Joseph Henry articulated a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This keynote of basic research has been adhered to over the years in the issuance of thousands of titles in serial publications under the Smithsonian imprint, com- mencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Annals of Flight Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in History and Technology In these series, the Institution publishes original articles and monographs dealing with the research and collections of its several museums and offices and of professional colleagues at other institutions of learning. These papers report newly acquired facts, synoptic interpretations of data, or original theory in specialized fields. These pub- lications are distributed by mailing lists to libraries, laboratories, and other interested institutions and specialists throughout the world. Individual copies may be obtained from the Smithsonian Institution Press as long as stocks are available. -

Table of Contents
TABLE OF CONTENTS SESSION ONE – PLENARY SESSION ............................................................................................... 1 CHAIRS: MICHAEL HODDA & DAVID CHITWOOD Is Nematology a Jigsaw, a Tapestry or a Strange Attractor? 1 Hodda, M. Metagenomics, Big Science, and the Reformation of Nematology 2 Powers, T. A Practical Future for Nematology in the Real World 2 Nicol, J. & R. Sikora SESSION TWO – ECOLOGY AND BIODIVERSITY OF SOIL NEMATODES IN SUSTAINABLE SOIL CONSERVATION ............................................................................................ 3 CONVENORS: GREGOR YEATES & NIGEL BELL Nematode Assemblages and Soil Properties Are Closely Linked 3 Sánchez-Moreno, S. & H. Ferris Nematode Diversity and Function in Dutch Sand Dunes 4 Brinkman, E.P., H. Duyts & W.H. Van der Putten Nematode Diversity under Commercial Banana Production 5 Pattison, A., J. Cobon, M. Araya, L. Pocasangre, F. Rosales & R. Sikora How Different or Similar are Nematode Communities in Paddy and Upland Rice Fields 6 Okada, H., W. Abe, M. Komatsuzaki & M. Hiroki A Perspective on Diversity within Nematode Feeding Groups across Ecosystems 7 Yeates, G.W. SESSION THREE – MUTUALISTIC/PHORETIC ASSOCIATIONS AND INVERTEBRATE PARASITIC NEMATODES ............................................................................................................... 8 CONVENORS: ROBIN GIBLIN-DAVIS & KERRIE DAVIES Entomophilic Nematodes for Predictions of Worldwide Nematode Species Diversity 8 Giblin-Davis, R.M., N. Kanzaki & K.A. Davies Host Specificity, -

Longidoridae and Trichodoridae (Nematoda: Dorylaimida and Triplonchida). Lincoln, N.Z.: Landcare Research
2 Xu & Zhao (2019) Longidoridae and Trichodoridae (Nematoda: Dorylaimida and Triplonchida) EDITORIAL BOARD Dr M. J. Fletcher, NSW Agricultural Scientific Collections Unit, Forest Road, Orange, NSW 2800, Australia Prof. G. Giribet, Museum of Comparative Zoology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, U.S.A. Dr R. J. B. Hoare, Manaaki Whenua - Landcare Research, Private Bag 92170, Auckland, New Zealand Mr R. L. Palma, Museum of New Zealand Te Papa Tongarewa, P.O. Box 467, Wellington, New Zealand Dr C. J. Vink, Canterbury Museum, Rolleston Ave, Christchurch, New Zealand CHIEF EDITOR Prof Z.-Q. Zhang, Manaaki Whenua - Landcare Research, Private Bag 92170, Auckland, New Zealand Associate Editors Dr T. R. Buckley, Dr R. J. B. Hoare, Dr R. A. B. Leschen, Dr D. F. Ward, Dr Z. Q. Zhao, Manaaki Whenua - Landcare Research, Private Bag 92170, Auckland, New Zealand Honorary Editor Dr T. K. Crosby, Manaaki Whenua - Landcare Research, Private Bag 92170, Auckland, New Zealand Fauna of New Zealand 79 3 Fauna of New Zealand Ko te Aitanga Pepeke o Aotearoa Number / Nama 79 Longidoridae and Trichodoridae (Nematoda: Dorylaimida and Triplonchida) by Yu-Mei Xu Agronomy College, Shanxi Agricultural University, Taigu 030801, China Email: [email protected] Zeng-Qi Zhao Manaaki Whenua - Landcare Research, 231 Morrin Road, Auckland, New Zealand Email: [email protected] Auckland, New Zealand 2019 4 Xu & Zhao (2019) Longidoridae and Trichodoridae (Nematoda: Dorylaimida and Triplonchida) Copyright © Manaaki Whenua - Landcare Research New Zealand Ltd 2019 No part of this work covered by copyright may be reproduced or copied in any form or by any means (graphic, elec- tronic, or mechanical, including photocopying, recording, taping information retrieval systems, or otherwise) without the written permission of the publisher. -

Some New and Known Species of Genera Tripylina Brzeski and Trischistoma Cobb, 1913 (Nematoda) with a Discussion on Their Relationships
Journal of Nematology 42(2):128–138. 2010. Ó The Society of Nematologists 2010. Some new and known species of genera Tripylina Brzeski and Trischistoma Cobb, 1913 (Nematoda) with a Discussion on their Relationships 1* 1 QUDSIA TAHSEEN, TABINDA NUSRAT Abstract: The paper deals with description of three new and two known species of Tripylidae O¨ rley, 1880. Two new and a known species of the genus Tripylina Brzeski, 1963 while one new and one known species of Trischistoma are described. Tripylina ymyensis n. sp. is characterised by robust body; long, slender outer labial setae; stoma with thick cuticularised wall bearing a prominent dorsal tooth and two equal-sized posteriorly-placed subventral teeth; large ovoid coelomocytes with conspicuous, globular inclusions; simple vagina without cuticularised pieces and a long, curved tail with a U- or S-shaped turn, uniformly tapering into a narrow terminus. Tripylina valiathani n. sp. is characterised by moderately large body; plump, leaf-shaped outer labial setae; stoma with thick cuticularised dorsal wall bearing a prominent dorsal tooth and two small anteriorly-placed subventral denticles; large fusiform coelomocytes with tapering ends having fine granular inclusions; cuticularised vagina, a distinct prerectum and a stumpy, laterally curved tail with a blunt terminus. T. stramenti (Yeates, 1972) Tsalolikhin, 1983 has been reported for the first time from India. Trischistoma minor n. sp. is characterised by medium-sized body; with dense crystalloids and granular aggregates; low, flattened lip region; plump outer labial setae; slenderer post-labial cephalic setae lying closely posterior to outer labials; a post-uterine sac of one body diameter and a dorsally curved tail with ventral terminal turn. -
Nematode Diversity of Native Species of Vitis in California
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 1-1996 Nematode Diversity of Native Species of Vitis in California Luma Al-Banna University of Jordan, [email protected] Scott Lyell Gardner University of Nebraska - Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Al-Banna, Luma and Gardner, Scott Lyell, "Nematode Diversity of Native Species of Vitis in California" (1996). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 65. https://digitalcommons.unl.edu/parasitologyfacpubs/65 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 971 Nematode diversity of native species of Vilis in California Luma AI Banna and Scott Lyell Gardner Abstract: From 1990 through 1992, nematodes were extracted from soil samples taken from the rhizosphere of native species of grapes from four areas of northern California and two areas of southern California. For comparison, samples from domestic grapes as well as a putative hybrid of Vitis califarnica and V. vinifera were also taken. Rhizosoil from California native grapevine contained many more species of nematodes than did soil obtained from cultivated forms of V. vinifera. Taxonomic and trophic diversity was much higher in nematodes from sampling sites from native grapes than in those from grapes maintained in vineyard situations. -

JOURNAL of NEMATOLOGY Morphological and Molecular
JOURNAL OF NEMATOLOGY Article | DOI: 10.21307/jofnem-2021-048 e2021-48 | Vol. 53 Morphological and molecular characterisation of Tripylina gorganensis from the Slovak Republic as a contribution to the redescription of the species Marek Renčo1,*, Katarzyna Rybarczyk-Mydłowska2, Łukasz Flis2, Magdalena Kubicz2 Abstract 2 and Grażyna Winiszewska Specimens of Tripylina gorganensis, collected from a natural beech 1Institute of Parasitology, Slovak forest in Slovak Republic, are described and illustrated. These Academy of Sciences, Hlinkova 3, nematodes were initially identified as an undescribed species, Košice, Slovak Republic. morphologically similar to Tripylina gorganensis described from Iran. An important feature distinguishing both species was the presence 2Museum and Institute of Zoology of post-vulval uterine sac (PUS) in specimens from Slovak Republic, PAS, Wilcza 64, 00-679 Warsaw, which, according to the original description (Asghari et al., 2012), was Poland. absent in Tripylina gorganensis. However, a careful re-examination of *E-mail: [email protected] type specimens performed in this study revealed that T. gorganensis also has the post-vulval uterine sac. Consequently, the findings of All authors contributed equally to the morphological and molecular studies performed on the Slovak this study. population and observations on the type material contribute to the This paper was edited by redescription of T. gorganensis. Thomas Powers. Received for publication Keywords October 14, 2020. Redescription, Fagus sylvatica, Molecular, Morphology, -

Impact of Trophic Ecologies on the Whereabouts of Nematodes in Soil
Impact of trophic ecologies on the whereabouts of nematodes in soil Casper W. Quist Thesis committee Promotor Prof. Dr Jaap Bakker Professor of Nematology Wageningen University & Research Co-promotor Dr Johannes Helder Associate professor, Laboratory of Nematology Wageningen University & Research Other members Prof. Dr Wietse de Boer, Wageningen University & Research Dr Sofie Derycke, Royal Belgian Institute of Natural Sciences, Brussels, Belgium Prof. Dr Michael Bonkowski, University of Cologne, Germany Dr Christien Ettema, CEO Shades of Green This research was conducted under the auspices of the C.T. de Wit Graduate School of Production Ecology and Resource Conservation Impact of trophic ecologies on the whereabouts of nematodes in soil Casper W. Quist Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus, Prof. Dr A.P.J Mol, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Friday 19 May 2017 at 1:30 p.m. in the Aula. Casper W. Quist Impact of trophic ecologies on the whereabouts of nematodes in soil, 130 pages. PhD thesis, Wageningen University, Wageningen, the Netherlands (2017) With references, with summary in English. ISBN 978-94-6343-081-4 DOI http://dx.doi.org/ 10.18174/403954 Contents Chapter 1 General introduction 7 Chapter 2 Selective alteration of soil food web components by 17 invasive giant goldenrod Solidago gigantea in two distinct habitat types (published in Oikos 2014, 123, 837-845) -

System Analysis and Nematode Phylogeny
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 1-1970 System Analysis and Nematode Phylogeny Armand R. Maggenti University of California - Davis Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Maggenti, Armand R., "System Analysis and Nematode Phylogeny" (1970). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 102. https://digitalcommons.unl.edu/parasitologyfacpubs/102 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. J Nematol. 1970 January; 2(1): 7–15. System Analysis and Nematode Phylogeny A. R. MAGGENTI 1 The purpose here is to suggest a method The available fossils represent only a small which will permit understanding of the fraction of the former plant and animal life phylogenetic relationships of contemporary on earth. That enormous numbers of animals representatives of the phylum Nematoda and plants, important to the evolution of without fossils. It is not my intent to again contemporary forms, must have existed and (14) offer a phylogeny for the Nematoda. declined to extinction without leaving a trace To say that no real benefit or knowledge of is illustrated by an observation in recent phylogeny or evolution is possible without historical times. Of the millions of buffalo fossils is succumbing to apathy.