The Abused Inhalant Toluene Differentially Modulates Excitatory and Inhibitory Synaptic Transmission in Deep-Layer Neurons of the Medial Prefrontal Cortex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review of Market for Octane Enhancers
May 2000 • NREL/SR-580-28193 Review of Market for Octane Enhancers Final Report J.E. Sinor Consultants, Inc. Niwot, Colorado National Renewable Energy Laboratory 1617 Cole Boulevard Golden, Colorado 80401-3393 NREL is a U.S. Department of Energy Laboratory Operated by Midwest Research Institute • Battelle • Bechtel Contract No. DE-AC36-99-GO10337 May 2000 • NREL/SR-580-28193 Review of Market for Octane Enhancers Final Report J.E. Sinor Consultants, Inc. Niwot, Colorado NREL Technical Monitor: K. Ibsen Prepared under Subcontract No. TXE-0-29113-01 National Renewable Energy Laboratory 1617 Cole Boulevard Golden, Colorado 80401-3393 NREL is a U.S. Department of Energy Laboratory Operated by Midwest Research Institute • Battelle • Bechtel Contract No. DE-AC36-99-GO10337 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof. Available electronically at http://www.doe.gov/bridge Available for a processing fee to U.S. -

Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances 2017
INTERNATIONAL NARCOTICS CONTROL BOARD Precursors and chemicals frequently used in the illicit manufacture of narcotic drugs and psychotropic substances 2017 EMBARGO Observe release date: Not to be published or broadcast before Thursday, 1 March 2018, at 1100 hours (CET) UNITED NATIONS CAUTION Reports published by the International Narcotics Control Board in 2017 The Report of the International Narcotics Control Board for 2017 (E/INCB/2017/1) is supplemented by the following reports: Narcotic Drugs: Estimated World Requirements for 2018—Statistics for 2016 (E/INCB/2017/2) Psychotropic Substances: Statistics for 2016—Assessments of Annual Medical and Scientific Requirements for Substances in Schedules II, III and IV of the Convention on Psychotropic Substances of 1971 (E/INCB/2017/3) Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances: Report of the International Narcotics Control Board for 2017 on the Implementation of Article 12 of the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 (E/INCB/2017/4) The updated lists of substances under international control, comprising narcotic drugs, psychotropic substances and substances frequently used in the illicit manufacture of narcotic drugs and psychotropic substances, are contained in the latest editions of the annexes to the statistical forms (“Yellow List”, “Green List” and “Red List”), which are also issued by the Board. Contacting the International Narcotics Control Board The secretariat of the Board may be reached at the following address: Vienna International Centre Room E-1339 P.O. Box 500 1400 Vienna Austria In addition, the following may be used to contact the secretariat: Telephone: (+43-1) 26060 Fax: (+43-1) 26060-5867 or 26060-5868 Email: [email protected] The text of the present report is also available on the website of the Board (www.incb.org). -

Organocatalytic Asymmetric N-Sulfonyl Amide C-N Bond Activation to Access Axially Chiral Biaryl Amino Acids
ARTICLE https://doi.org/10.1038/s41467-020-14799-8 OPEN Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids Guanjie Wang1, Qianqian Shi2, Wanyao Hu1, Tao Chen1, Yingying Guo1, Zhouli Hu1, Minghua Gong1, ✉ ✉ ✉ Jingcheng Guo1, Donghui Wei 2 , Zhenqian Fu 1,3 & Wei Huang1,3 1234567890():,; Amides are among the most fundamental functional groups and essential structural units, widely used in chemistry, biochemistry and material science. Amide synthesis and trans- formations is a topic of continuous interest in organic chemistry. However, direct catalytic asymmetric activation of amide C-N bonds still remains a long-standing challenge due to high stability of amide linkages. Herein, we describe an organocatalytic asymmetric amide C-N bonds cleavage of N-sulfonyl biaryl lactams under mild conditions, developing a general and practical method for atroposelective construction of axially chiral biaryl amino acids. A structurally diverse set of axially chiral biaryl amino acids are obtained in high yields with excellent enantioselectivities. Moreover, a variety of axially chiral unsymmetrical biaryl organocatalysts are efficiently constructed from the resulting axially chiral biaryl amino acids by our present strategy, and show competitive outcomes in asymmetric reactions. 1 Key Laboratory of Flexible Electronics & Institute of Advanced Materials, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, China. 2 College -
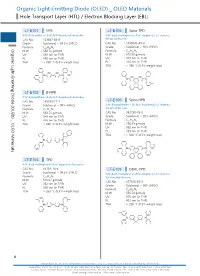
Organic Light-Emitting Diode (OLED) OLED Materials Hole Transport Layer (HTL) / Electron Blocking Layer (EBL)
Organic Light-EmittingOrganic Light-Emitting DiodeHole Transport(OLED) Layer (HTL) / Diode (OLED) _ OLED Materials Electron Blocking Layer (EBL) Organic Light-Emitting Diode (OLED) _ OLED Materials Hole Transport Layer (HTL) / Electron Blocking Layer (EBL) LT-E101 NPB LT-E105 Spiro-TPD N,N'-Bis(naphthalen-1-yl)-N,N'-bis(phenyl)-benzidine N,N'-Bis(3-methylphenyl)-N,N'-bis(phenyl)-2,7-diamino- CAS No. : 123847-85-8 9,9-spirobifluorene Grade : Sublimed, > 99.5% (HPLC) CAS No. : 1033035-83-4 : Grade : Sublimed, > 99% (HPLC) Organic Light-Emitting Diode (OLED) _ OLED Materials Formula C44H32N2 M.W. : 588.74 g/mole Formula : C51H38N2 UV : 339 nm (in THF) M.W. : 678.86 g/mole PL : 450 nm (in THF) UV : 379 nm (in THF) TGA : > 350 °C (0.5% weight loss) PL : 416 nm (in THF) TGA : > 280 °C (0.5% weight loss) N N N N LT-E102 β-NPB N,N'-Bis(naphthalen-2-yl)-N,N'-bis(phenyl)-benzidine Spiro-NPB CAS No. : 139255-17-7 LT-E106 Grade : Sublimed, > 99% (HPLC) N,N'-Bis(naphthalen-1-yl)-N,N'-bis(phenyl)-2,7-diamino- 9,9-spirobifluorene Formula : C44H32N2 M.W. : 588.74 g/mole CAS No. : 932739-76-9 UV : 349 nm (in THF) Grade : Sublimed, > 99% (HPLC) PL : 416 nm (in THF) Formula : C57H38N2 TGA : > 330 °C (0.5% weight loss) M.W. : 750.93 g/mole UV : 380 nm (in THF) PL : 453 nm (in THF) TGA : > 360 °C (0.5% weight loss) N N N N LT-E103 TPD N,N'-Bis(3-methylphenyl)-N,N'-bis(phenyl)-benzidine CAS No. -

Both N-Methyl-D-Aspartate and Non-N-Methyl- D-Aspartate Glutamate Receptors in the Bed
JOP0010.1177/0269881117691468Journal of PsychopharmacologyAdami et al. 691468research-article2017 Original Paper Both N-methyl-D-aspartate and non-N-methyl- D-aspartate glutamate receptors in the bed nucleus of the stria terminalis modulate the Journal of Psychopharmacology 2017, Vol. 31(6) 674 –681 cardiovascular responses to acute restraint © The Author(s) 2017 Reprints and permissions: sagepub.co.uk/journalsPermissions.nav stress in rats DOI:https://doi.org/10.1177/0269881117691468 10.1177/0269881117691468 journals.sagepub.com/home/jop Mariane B Adami1*, Lucas Barretto-de-Souza1,2*, Josiane O Duarte1, Jeferson Almeida1,2 and Carlos C Crestani1,2 Abstract The bed nucleus of the stria terminalis (BNST) is a forebrain structure that has been implicated on cardiovascular responses evoked by emotional stress. However, the local neurochemical mechanisms mediating the BNST control of stress responses are not fully described. In our study we investigated the involvement of glutamatergic neurotransmission within the BNST in cardiovascular changes evoked by acute restraint stress in rats. For this study, we investigated the effects of bilateral microinjections of selective antagonists of either N-methyl-D-aspartate (NMDA) or non-NMDA glutamate receptors into the BNST on the arterial pressure and heart rate increase and the decrease in tail skin temperature induced by acute restraint stress. Microinjection of the selective NMDA glutamate receptor antagonist LY235959 (1 nmol/100 nL) into the BNST decreased the tachycardiac response to restraint stress, without affecting the arterial pressure increase and the drop in skin temperature. Bilateral BNST treatment with the selective non-NMDA glutamate receptor NBQX (1 nmol/100 nL) decreased the heart rate increase and the fall in tail skin temperature, without affecting the blood pressure increase. -

Hydroxydopamine Lesions of the Nucleus Accumbens and the Mglur2/3 Agonist LY379268
Neuropsychopharmacology (2003) 28, 1440–1447 & 2003 Nature Publishing Group All rights reserved 0893-133X/03 $25.00 www.neuropsychopharmacology.org Toluene-Induced Locomotor Activity is Blocked by 6- Hydroxydopamine Lesions of the Nucleus Accumbens and the mGluR2/3 Agonist LY379268 1,3 2 ,1 AC Riegel , SF Ali , ED French* 1Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ, USA; 2Neurochemistry Laboratory, Division of Neurotoxicology, National Center for Toxicological Research/US FDA, Jefferson, AR, USA The abuse of volatile inhalants remains a prominent, yet poorly understood, form of substance abuse among youth. Nevertheless, the identification of a mechanism underlying the reinforcing properties of inhalants has been hampered by the lack of a clearly identifiable neural substrate upon which these chemicals act. One ingredient that is common to many abused inhalants is toluene, an organic solvent that is self-administered by nonhuman primates and rodents. Most drugs of abuse have been found to elicit forward locomotion in rats, an effect owing to the activation of mesoaccumbal dopamine (DA) pathways. Thus, the present study was undertaken using two different approaches to determine whether toluene-induced locomotor hyperactivity is also ultimately dependent upon DA neurotransmission in the mesolimbic nucleus accumbens (NAC). Here we report on the effects of 6-hydroxydopamine (6-OHDA) lesions of the NAC or pretreatment with the metabotropic mGlu2/3 receptor agonist LY379268 on toluene-induced locomotor activity. Both procedures, which are known to alter neurotransmission within the NAC, significantly attenuated toluene’s locomotor stimulatory effects. These results provide strong support for a central mechanism of action of inhalants, which in the past has been more typically attributed to general nonspecific mechanisms throughout the brain. -

Thyroid Hormones Modulate GABAA Receptor-Mediated Currents in Hippocampal Neurons
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archivio istituzionale della ricerca - Università di Modena e Reggio Emilia Neuropharmacology 60 (2011) 1254e1261 Contents lists available at ScienceDirect Neuropharmacology journal homepage: www.elsevier.com/locate/neuropharm Thyroid hormones modulate GABAA receptor-mediated currents in hippocampal neurons G. Puia*, G. Losi 1 Department of Biomedical Science, Pharmacology Section, University of Modena and Reggio Emilia, via Campi 287, 41100 Modena, Italy article info abstract Article history: Thyroid hormones (THs) play a crucial role in the maturation and functioning of mammalian central 0 Received 4 August 2010 nervous system. Thyroxine (T4) and 3, 3 ,5-L-triiodothyronine (T3) are well known for their genomic Received in revised form effects, but recently attention has been focused on their non genomic actions as modulators of neuronal 28 October 2010 activity. In the present study we report that T4 and T3 reduce, in a non competitive manner, GABA- Accepted 15 December 2010 evoked currents in rat hippocampal cultures with IC50sof13Æ 4 mM and 12 Æ 3 mM, respectively. The genomically inactive compound rev-T3 was also able to inhibit the currents elicited by GABA. Blocking Keywords: PKC or PKA activity, chelating intracellular calcium, or antagonizing the integrin receptor aVb3 with Thryoid hormones GABAergic neurotransmission TETRAC did not affect THs modulation of GABA-evoked currents. THs affect also synaptic activity in Neuronal cultures hippocampal and cortical cultured neurons. sIPSCs T3 and T4 reduced to approximately 50% the amplitude and frequency of spontaneous inhibitory Tonic currents synaptic currents (sIPSCs), without altering their decay kinetic. -

Inhalant Abuse Pediatric Care
CLINICAL REPORT Guidance for the Clinician in Rendering Inhalant Abuse Pediatric Care Janet F. Williams, MD, Michael Storck, MD, and the Committee on Substance Abuse and Committee on Native American Child Health ABSTRACT Inhalant abuse is the intentional inhalation of a volatile substance for the purpose of achieving an altered mental state. As an important, yet-underrecognized form of substance abuse, inhalant abuse crosses all demographic, ethnic, and socioeco- nomic boundaries, causing significant morbidity and mortality in school-aged and older children. This clinical report reviews key aspects of inhalant abuse, empha- sizes the need for greater awareness, and offers advice regarding the pediatrician’s role in the prevention and management of this substance abuse problem. TYPES OF CHEMICALS AND PRODUCTS ABUSED The term “inhalant” encompasses a wide range of pharmacologically diverse substances that readily vaporize. Most other substances of abuse are classified by grouping together substances that share a specific central nervous system action or perceived psychoactive effect, but inhalant substances that are abused are grouped by having a common route of drug use. Inhalant abuse, sometimes referred to as solvent or volatile substance abuse, can be better understood when the expansive list of inhalants is classified into 3 groups on the basis of what is currently known pharmacologically: group I includes volatile solvents, fuels, and anesthetics; group II includes nitrous oxide; and group III includes volatile alkyl nitrites (Table 1). This classification is also consistent with reported differences in user populations, patterns of abuse, and associated problems seen clinically.1–3 Drugs that do not www.pediatrics.org/cgi/doi/10.1542/ readily vaporize at room temperature, such as cocaine, heroin, nicotine, or alcohol, peds.2007-0470 can also be abused through inhalation, but characteristic pharmacologic properties doi:10.1542/peds.2007-0470 distinguish these substances from inhalants. -

NBQX Attenuates Excitotoxic Injury in Developing White Matter
The Journal of Neuroscience, December 15, 2000, 20(24):9235–9241 NBQX Attenuates Excitotoxic Injury in Developing White Matter Pamela L. Follett, Paul A. Rosenberg, Joseph J. Volpe, and Frances E. Jensen Department of Neurology and Program in Neuroscience, Children’s Hospital and Harvard Medical School, Boston, Massachusetts 02115 The excitatory neurotransmitter glutamate is released from axons hr) resulted in selective, subcortical white matter injury with a and glia under hypoxic/ischemic conditions. In vitro, oligoden- marked ipsilateral decrease in immature and myelin basic drocytes (OLs) express non-NMDA glutamate receptors (GluRs) protein-expressing OLs that was also significantly attenuated by and are susceptible to GluR-mediated excitotoxicity. We evalu- 6-nitro-7-sulfamoylbenzo(f)quinoxaline-2,3-dione (NBQX). Intra- ated the role of GluR-mediated OL excitotoxicity in hypoxic/ cerebral AMPA demonstrated greater susceptibility to OL injury ischemic white matter injury in the developing brain. Hypoxic/ at P7 than in younger or older pups, and this was attenuated by ischemic white matter injury is thought to mediate periventricular systemic pretreatment with the AMPA antagonist NBQX. These leukomalacia, an age-dependent white matter lesion seen in results indicate a parallel, maturation-dependent susceptibility of preterm infants and a common antecedent to cerebral palsy. immature OLs to AMPA and hypoxia/ischemia. The protective Hypoxia/ischemia in rat pups at postnatal day 7 (P7) produced efficacy of NBQX suggests a role for glutamate receptor- selective white matter lesions and OL death. Furthermore, OLs in mediated excitotoxic OL injury in immature white matter in vivo. pericallosal white matter express non-NMDA GluRs at P7. -

NIH Public Access Author Manuscript Addict Biol
NIH Public Access Author Manuscript Addict Biol. Author manuscript; available in PMC 2014 January 01. Published in final edited form as: Addict Biol. 2013 January ; 18(1): 54–65. doi:10.1111/adb.12000. Enhanced AMPA Receptor Activity Increases Operant Alcohol Self-administration and Cue-Induced Reinstatement $watermark-text $watermark-text $watermark-text Reginald Cannadya,b, Kristen R. Fishera, Brandon Duranta, Joyce Besheera,b,c, and Clyde W. Hodgea,b,c,d aBowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599 bCurriculum in Neurobiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599 cDepartment of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599 dDepartment of Pharmacology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599 Abstract Long-term alcohol exposure produces neuroadaptations that contribute to the progression of alcohol abuse disorders. Chronic alcohol consumption results in strengthened excitatory neurotransmission and increased AMPA receptor signaling in animal models. However, the mechanistic role of enhanced AMPA receptor activity in alcohol reinforcement and alcohol- seeking behavior remains unclear. This study examined the role of enhanced AMPA receptor function using the selective positive allosteric modulator, aniracetam, in modulating operant alcohol self-administration and cue-induced reinstatement. Male alcohol-preferring (P-) rats, trained to self-administer alcohol (15%, v/v) versus water were pretreated with aniracetam to assess effects on maintenance of alcohol self-administration. To determine reinforcer specificity, P-rats were trained to self-administer sucrose (0.8%, w/v) versus water, and effects of aniracetam were tested. -

The Effects of the Morphine Analogue Levorphanol on Leukocytes: Metabolic Effects at Rest and During Phagocytosis
The effects of the morphine analogue levorphanol on leukocytes: Metabolic effects at rest and during phagocytosis Nancy Wurster, … , Penelope Pettis, Sharon Lebow J Clin Invest. 1971;50(5):1091-1099. https://doi.org/10.1172/JCI106580. Studies on bacteria have suggested that morphine-like drugs have effects on the cell membrane. To determine the effect of this class of drugs on a mammalian cell, we selected the rabbit peritoneal exudate granulocyte, which undergoes striking membrane changes during phagocytosis. We examined the effect in vitro of the morphine analogue, levorphanol on phagocytosis and metabolism by granulocytes incubated with and without polystyrene particles or live Escherichia coli. Levorphanol (1 or 2 mmoles/liter) decreased: (a) acylation of lysolecithin or lysophosphatidylethanolamine in the medium (which is stimulated about two-fold during phagocytosis) both at rest (40%) and during phagocytosis (60%); (b) uptake of latex particles and Escherichia coli, as judged by electron microscopy; (c) killing of live Escherichia coli (10-fold); (d) 14 14 + CO2 production from glucose-1- C during phagocytosis by at least 80%; (e) K content of granulocytes (35%); (f) oxidation of linoleate-1-14C by 50%, and its incorporation into triglyceride by more than 80%. However, levorphanol stimulated 2 to 3-fold the incorporation of linoleate-1-14C or palmitate-1-14C into several phospholipids. Glucose uptake, lactate production, and adenosine triphosphate (ATP) content are not affected by the drug. Thus, levorphanol does not appear to exert its effects through generalized metabolic suppression. Removal of levorphanol by twice resuspending the granulocytes completely reverses all inhibition. In line with observations on bacteria, it appears that the complex effects of levorphanol on […] Find the latest version: https://jci.me/106580/pdf The Effects of the Morphine Analogue Levorphanol on Leukocytes METABOLIC EFFECTS AT REST AND DURING PHAGOCYTOSIS NANcY WuRsTE, PETER ELSBACH, ERIc J. -

Homomeric Q/R Edited AMPA Receptors Conduct When Desensitized Ian D
bioRxiv preprint doi: https://doi.org/10.1101/595009; this version posted April 1, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Homomeric Q/R edited AMPA receptors conduct when desensitized Ian D. Coombs1, David Soto1, 2, Thomas P. McGee1, Matthew G. Gold1, Mark Farrant*1, and Stuart G. Cull-Candy*1 1Department of Neuroscience, Physiology and Pharmacology, University College London, Gower Street, London WC1E 6BT, UK; 2Present Address – Department of Biomedicine, Neurophysiology Laboratory, Medical School, Institute of Neurosciences, University of Barcelona, Casanova 143, 08036 Barcelona, Spain. Correspondence to [email protected] or [email protected] Desensitization is a canonical property of ligand-gated ion channels, causing progressive current decline in the continued presence of agonist. AMPA-type glutamate receptors, which mediate fast excitatory sig- naling throughout the brain, exhibit profound desensitization. Recent cryo-EM studies of AMPAR assem- blies show their ion channels to be closed in the desensitized state. Here we report the surprising finding that homomeric Q/R edited AMPARs still allow ions to flow when the receptors are desensitized. GluA2(R) expressed alone, or with auxiliary subunits (γ-2, γ-8 or GSG1L), generates large steady-state currents and anomalous current-variance relationships. Using fluctuation analysis, single-channel recording, and kinetic modeling we demonstrate that the steady-state current is mediated predominantly by ‘conducting desensitized’ receptors.