Journal of Rese Arch in B Iolog Y
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

District Wise Skill Gap Study for the State of Haryana.Pdf
District wise skill gap study for the State of Haryana Contents 1 Report Structure 4 2 Acknowledgement 5 3 Study Objectives 6 4 Approach and Methodology 7 5 Growth of Human Capital in Haryana 16 6 Labour Force Distribution in the State 45 7 Estimated labour force composition in 2017 & 2022 48 8 Migration Situation in the State 51 9 Incremental Manpower Requirements 53 10 Human Resource Development 61 11 Skill Training through Government Endowments 69 12 Estimated Training Capacity Gap in Haryana 71 13 Youth Aspirations in Haryana 74 14 Institutional Challenges in Skill Development 78 15 Workforce Related Issues faced by the industry 80 16 Institutional Recommendations for Skill Development in the State 81 17 District Wise Skill Gap Assessment 87 17.1. Skill Gap Assessment of Ambala District 87 17.2. Skill Gap Assessment of Bhiwani District 101 17.3. Skill Gap Assessment of Fatehabad District 115 17.4. Skill Gap Assessment of Faridabad District 129 2 17.5. Skill Gap Assessment of Gurgaon District 143 17.6. Skill Gap Assessment of Hisar District 158 17.7. Skill Gap Assessment of Jhajjar District 172 17.8. Skill Gap Assessment of Jind District 186 17.9. Skill Gap Assessment of Kaithal District 199 17.10. Skill Gap Assessment of Karnal District 213 17.11. Skill Gap Assessment of Kurukshetra District 227 17.12. Skill Gap Assessment of Mahendragarh District 242 17.13. Skill Gap Assessment of Mewat District 255 17.14. Skill Gap Assessment of Palwal District 268 17.15. Skill Gap Assessment of Panchkula District 280 17.16. -

SHORT NO Sealed Tenders for the Following Wo
HARYANA TOURISM SHORT NOTICE INVITING TENDERS Sealed tenders for the following work is hereby invited by the Executive Engineer, Haryana Tourism Corporation, Chandigarh from approved contractors & consultants of Haryana PWD (B&R) / Haryana Tourism /PHED/ HUDA / CPWD / MES / Railways / HSAMB / HPHC or any other State / Central Government Departments, Boards / Corporation of any States or GOI. Tender must be accompanied with the Earnest money in the shape of Demand Draft, in the name of Executive Engineer Haryana Tourism Corporation, payable at Chandigarh. The tender will be opened on the dates shown against each in the presence of contractor or their authorized agents / representative who may like to be present at that time. If the date of opening of tenders happens to be a holiday then the tenders will be opened on the next working day. The drawings and DNIT can be seen in the office of the Executive Engineer, Haryana Tourism Corporation, SCO 17-19, Sector-17 B, Chandigarh.( Telephone no. 0172- 2727829) or visit our website www.haryanatourism.gov.in. Prescribed tender form may be obtained from the office of the Executive Engineer, Haryana Tourism Corporation against cash payment of Rs.500/- (Non refundable) on any working day. The Earnest Money will be shown / deposited at the time of issue of tender form. Sr.No Name of work Estimated Earnest Time Last Date Date & Time Cost Money limit of issue of of Opening (in lacs) tender form (upto 3.30 (upto 4.30 P.M.) P.M.) 1. Development of 4.15 Rs.8300/- 1 Month 11.07.2016 12.07.2016 Yamunanagar-Panchkula- Poanta Sahib as a Mega Tourism Circuit (landscaping and plantation of Gymkhana Club , HUDA, Jagadhari). -

Directory of Officers Office of Director of Income Tax (Inv.) Chandigarh Sr
Directory of Officers Office of Director of Income Tax (Inv.) Chandigarh Sr. No. Name of the Officer Designation Office Address Contact Details (Sh./Smt./Ms/) 1 P.S. Puniha DIT (Inv.) Room No. - 201, 0172-2582408, Mob - 9463999320 Chandigarh Aayakar Bhawan, Fax-0172-2587535 Sector-2, Panchkula e-mail - [email protected] 2 Adarsh Kumar ADIT (Inv.) (HQ) Room No. - 208, 0172-2560168, Mob - 9530765400 Chandigarh Aayakar Bhawan, Fax-0172-2582226 Sector-2, Panchkula 3 C. Chandrakanta Addl. DIT (Inv.) Room No. - 203, 0172-2582301, Mob. - 9530704451 Chandigarh Aayakar Bhawan, Fax-0172-2357536 Sector-2, Panchkula e-mail - [email protected] 4 Sunil Kumar Yadav DDIT (Inv.)-II Room No. - 207, 0172-2583434, Mob - 9530706786 Chandigarh Aayakar Bhawan, Fax-0172-2583434 Sector-2, Panchkula e-mail - [email protected] 5 SurendraMeena DDIT (Inv.)-I Room No. 209, 0172-2582855, Mob - 9530703198 Chandigarh Aayakar Bhawan, Fax-0172-2582855 Sector-2, Panchkula e-mail - [email protected] 6 Manveet Singh ADIT (Inv.)-III Room No. - 211, 0172-2585432 Sehgal Chandigarh Aayakar Bhawan, Fax-0172-2585432 Sector-2, Panchkula 7 Sunil Kumar Yadav DDIT (Inv.) Shimla Block No. 22, SDA 0177-2621567, Mob - 9530706786 Complex, Kusumpti, Fax-0177-2621567 Shimla-9 (H.P.) e-mail - [email protected] 8 Padi Tatung DDIT (Inv.) Ambala Aayakar Bhawan, 0171-2632839 AmbalaCantt Fax-0171-2632839 9 K.K. Mittal Addl. DIT (Inv.) New CGO Complex, B- 0129-24715981, Mob - 9818654402 Faridabad Block, NH-IV, NIT, 0129-2422252 Faridabad e-mail - [email protected] 10 Himanshu Roy ADIT (Inv.)-II New CGO Complex, B- 0129-2410530, Mob - 9468400458 Faridabad Block, NH-IV, NIT, Fax-0129-2422252 Faridabad e-mail - [email protected] 11 Dr.Vinod Sharma DDIT (Inv.)-I New CGO Complex, B- 0129-2413675, Mob - 9468300345 Faridabad Block, NH-IV, NIT, Faridabad e-mail - [email protected] 12 ShashiKajle DDIT (Inv.) Panipat SCO-44, Near Angel 0180-2631333, Mob - 9468300153 Mall, Sector-11, Fax-0180-2631333 Panipat e-mail - [email protected] 13 ShashiKajle (Addl. -

District Survey Report for Sustainable Sand Mining Distt. Yamuna Nagar
DISTRICT SURVEY REPORT FOR SUSTAINABLE SAND MINING DISTT. YAMUNA NAGAR The Boulder, Gravel and Sand are one of the most important construction materials. These minerals are found deposited in river bed as well as adjoining areas. These aggregates of raw materials are used in the highest volume on earth after water. Therefore, it is the need of hour that mining of these aggregates should be carried out in a scientific and environment friendly manner. In an endeavour to achieve the same, District Survey Report, apropos “the Sustainable Sand Mining Guidelines” is being prepared to identify the areas of aggradations or deposition where mining can be allowed; and identification of areas of erosion and proximity to infrastructural structural and installations where mining should be prohibited and calculation of annual rate of replenishment and allowing time for replenishment after mining in that area. 1. Introduction:- Minor Mineral Deposits: 1.1 Yamunanagar district of Haryana is located in north-eastern part of Haryana State and lies between 29° 55' to 30° 31 North latitudes and 77° 00' to 77° 35' East longitudes. The total area is 1756 square kilometers, in which there are 655 villages, 10 towns, 4 tehsils and 2 sub-tehsils. Large part of the district of Yamunanagar is situated in the Shiwalik foothills. The area of Yamuna Nagar district is bounded by the state of Himachal Pradesh in the north, by the state of Uttar Pradesh in the east, in west by Ambala district and south by Karnal and Kurukshetra Districts. 1.2 The district has a sub-tropical continental monsoon climate where we find seasonal rhythm, hot summer, cool winter, unreliable rainfall and immense variation in temperature. -

Haryana Highway Upgrading Project Project Coordinationconsultancy
PUBLIC WORKS DEPARTMENT (B&R) GOVERNMENT OF HARYANA, INDIA Public Disclosure Authorized HaryanaHighway Upgrading Project ProjectCoordination Consultancy SECTORALENVIRONMENTAL ASSESSMENT Public Disclosure Authorized FINAL REPORT CMA,,ISA \ Public Disclosure Authorized VOLUMEI11 APPENDICES SEPTEMBER 1997 Public Disclosure Authorized CarlBro Internationalals - (2,inassociation with BCEOM,Louis Berger International Inc. and J adBroGrot ConsultingEngineering Services (India)Ltd. PUBLIC WORKS DEPARTMENT (B&R) GOVERNMENT OF HARYANA, INDIA Haryana Highway Upgrading Project Project CoordinationConsultancy SECTORALENVIRONMENTAL ASSESSMENT FINALREPORT VOLUMEII APPENDICES SEPTEMBER1997 ~ CarlBro International als in associationwith BCEOM,Louis Berger International Inc. and J CarlBroGroup ConsultingEngineering Services (India) Ltd. VOLUME It - APPENDICES TO MAIN REPORT Number Appendix Page (s) Appendix I EnvironmentalAttributes of ROW corridors Al-I Appendix2 EnvironmentalStandards A2-1 Appendix3 Contract RelatedDocumentation A3-1 Appendix4 EnvironmentalManagement Checklist A4-1 Appendix5 EnvironmentalClauses to BiddingDocuments A5-1 Appendix6 List of Consultations A6-l tiaryana tiignway upgraing rrojecr A%lirivirunmentnai AFnnDUTCS 01 KU W APPENDIX 1 Environmental Attributes of ROW Corridors HaryanaHighwey Uprading Project Appendix I ENVIRONMENTALATTRIBUTES ON 20 KM CORRIDOR SEGMENT-2: SHAHZADPUR-SAHA(15.6 KM) ATrRIBUTES LOCATION & DESCRIPTION S.O.L Map Reference 53F/3 53B115 Topography Roadpase throughmore or lessplain are Erosional Features None shownin SOI mnap Water odies - AmnrChoaRiver at6km;Markandari sat0-10kmoff4km; Dhanaurrierat km0-16off7 :Badali iver at km 0-16 off5 krn; Dangri river at km 016 off 10km; Begnarive at ht 0-1 off4 km Natural Vegetation None shownin SOImnap Agriculture Road pas thruh cultivatedland on both sides Industry None shownti SOImaps Urban Settlement Shahz dpurTownship: At km 15; Saa township:at km 15 Communication None shownin SOI maps PowerUne Notshown in SOl maps Social Institution/Defence/Alrport Noneshown in SOI maps A. -
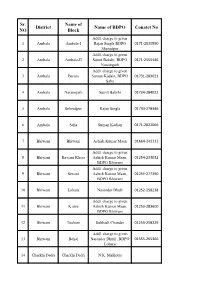
Sr. NO District Name of Block Name of BDPO Conatct No
Sr. Name of District Name of BDPO Conatct No NO Block Addl. charge to given 1 Ambala Ambala-I Rajan Singla BDPO 0171-2530550 Shazadpur Addl. charge to given 2 Ambala Ambala-II Sumit Bakshi, BDPO 0171-2555446 Naraingarh Addl. charge to given 3 Ambala Barara Suman Kadain, BDPO 01731-283021 Saha 4 Ambala Naraingarh Sumit Bakshi 01734-284022 5 Ambala Sehzadpur Rajan Singla 01734-278346 6 Ambala Saha Suman Kadian 0171-2822066 7 Bhiwani Bhiwani Ashish Kumar Maan 01664-242212 Addl. charge to given 8 Bhiwani Bawani Khera Ashish Kumar Maan, 01254-233032 BDPO Bhiwani Addl. charge to given 9 Bhiwani Siwani Ashish Kumar Maan, 01255-277390 BDPO Bhiwani 10 Bhiwani Loharu Narender Dhull 01252-258238 Addl. charge to given 11 Bhiwani K airu Ashish Kumar Maan, 01253-283600 BDPO Bhiwani 12 Bhiwani Tosham Subhash Chander 01253-258229 Addl. charge to given 13 Bhiwani Behal Narender Dhull , BDPO 01555-265366 Loharu 14 Charkhi Dadri Charkhi Dadri N.K. Malhotra Addl. charge to given 15 Charkhi Dadri Bond Narender Singh, BDPO 01252-220071 Charkhi Dadri Addl. charge to given 16 Charkhi Dadri Jhoju Ashok Kumar Chikara, 01250-220053 BDPO Badhra 17 Charkhi Dadri Badhra Jitender Kumar 01252-253295 18 Faridabad Faridabad Pardeep -I (ESM) 0129-4077237 19 Faridabad Ballabgarh Pooja Sharma 0129-2242244 Addl. charge to given 20 Faridabad Tigaon Pardeep-I, BDPO 9991188187/land line not av Faridabad Addl. charge to given 21 Faridabad Prithla Pooja Sharma, BDPO 01275-262386 Ballabgarh 22 Fatehabad Fatehabad Sombir 01667-220018 Addl. charge to given 23 Fatehabad Ratia Ravinder Kumar, BDPO 01697-250052 Bhuna 24 Fatehabad Tohana Narender Singh 01692-230064 Addl. -

Village & Townwise Primary Census Abstract, Yamunanagar, Part XII A
CENSUS OF INDIA 1991 SERIES -8 HARYANA DISTRICT CEN.SUS HANDBOOK PART XII - A & B VILLAGE & TOWN DIRECTORY VILLAGE &TOWNWISE PRIMARY CENSUS ABSTRACT DISTRICT YAMUNANAGAR Direqtor of Census Operations Haryana Published by : The Government of Haryana. 1995 ir=~~~==~==~==~====~==~====~~~l HARYANA DISTRICT YAMUNANAGAR t, :~ Km 5E3:::a::E0i:::=::::i====310==::::1i:5==~20. Km C.O.BLOCKS A SADAURA B BILASPUR C RADAUR o JAGADHRI E CHHACHHRAULI C.D.BLOCK BOUNDARY EXCLUDES STATUTORY TOWN (S) BOUNDARIES ARE UPDATED UPTO 1.1.1990 W. R.C. WORKSHOP RAILWAY COLONY DISTRICT YAMUNANAGAR CHANGE IN JURI50lC TION 1981-91 KmlO 0 10 Km L__.j___l BOUNDARY, STATE ... .. .. .. _ _ _ DISTRICT _ TAHSIL C D. BLOCK·:' .. HEADQUARTERS: DISTRICT; TAHSIL; e.D. BLOCK @:©:O STATE HIGHWAY.... SH6 IMPORT ANi MEiALLED ROAD RAILWAY LINE WITH STATION. BROAD GAUGE RS RIVER AND STREAMI CANAL ~/--- - Khaj,wan VILLAGE HAVING 5000 AND ABOVE POPULATION WITH NAME - URBAN AREA WITH POPULATION SIZE-CLASS I,II,IV &V .. POST AND TElEGRAPH OFFICE. PTO DEGREE COLLEGE AND TECHNICAL INSTITUTION ... ••••1Bl m BOUNDARY, STATE DISTRICT REST HOUSE, TRAVELLERS' BUNGALOW, FOREST BUNGALOW RH TB rB CB TA.HSIL AND CANAL BUNGALOW NEWLY CREATED DISTRICT YAMuNANAGAR Other villages having PTO/RH/TB/FB/CB, ~tc. are shown as .. .Damla HAS BEEN FORMED BY TRANSFERRING PTO AREA FROM :- Western Yamuna Canal W.Y.C. olsTRle T AMBAl,A I DISTRICT KURUKSHETRA SaSN upon Survt'y of India map with tn. p.rmission of theo Survt'yor Gf'nf'(al of India CENSUS OF INDIA - 1991 A - CENTRAL GOVERNMENT PUBLICATIONS The publications relating to Haryana bear series No. -

Brief Industrial Profile of Yamunanagar District
lR;eso t;rs Government of India Ministry of MSME Brief Industrial Profile of Yamunanagar District Carried out by:- MSME-Development Institute, Karnal (Ministry of MSME, Govt. of India) Phone: 0184-223082 Fax: 0184-2231862 e-mail: [email protected] Web- www.msmedikarnal.gov.in 1 Contents S. No. Topic Page No. 1. General Characteristics of the District 3 1.1 Location & Geographical Area 3 1.2 Topography 3 1.3 Availability of Minerals. 4 1.4 Forest 4 1.5 Administrative set up 4 2. District at a glance 5-7 2.1 Existing Status of Industrial Area in the District Yamunanagar 7 3. Industrial Scenario of District Yamunanagar 7-8 3.1 Industry at a Glance 8 3.2 Year Wise Trend Of Units Registered 8-9 3.3 Details Of Existing Micro & Small Enterprises & Artisan Units 9-10 In the District 3.4 Large Scale Industries / Public Sector undertakings 10 3.5 Major Exportable Item 11 3.6 Growth Trend 11 3.7 Vendorisation / Ancillarisation of the Industry 11 3.8 Medium Scale Enterprises 11 3.8.1 List of the units in Yamunanagar & near by Area 12 3.8.2 Major Exportable Item 12 3.9 Service Enterprises 12 3.9.1 Coaching Industry 12 3.9.2 Potentials areas for service industry 12 3.10 Potential for new MSMEs 12 4. Existing Clusters of Micro & Small Enterprise 12 4.1 Detail Of Major Clusters 12 4.1.1 Manufacturing Sector 12 4.1.2 Service Sector 13 4.2 Details of Identified cluster 13 4.2.1 Welding Electrodes - 4.2.2 Stone cluster - 4.2.3 Chemical cluster - 4.2.4 Fabrication and General Engg Cluster - 4.2. -

Clarification Regarding Architectural Control Sheets/Standard Designs
From: The Chief Administrator, HUDA, (Town Planning Wing) Panchkula. To 1. The Senior Town Planner,Panchkula, Gurgaon, Hissar 2. District Town Planner, Panchkula, Gurgaon, Faridabad, Hisar, Rohtak, Bahadurgarh,Karnal, Ambala, Kurukshetra, Sonepat, Panipat, Bhiwani, Jind, Sirsa, Rewari, Narnaul, Yamuna Nagar, Kaithal, Jhajjar, Fatehabad Memo no. CTP (H) 6778-6800 Dated: 29.12.05 Subject: Clarification regarding Architectural Control Shee ts/Standard Designs/Frame Controls. It has been observed that the Architectural Control Sheets/ Standard Designs and Frame Controls of Shopping, Public and Semi Public Bay Sites were prepared long ago. Since then, the requirement of public has undergone change and the internal layout Plan of the building can not be followed rigidly as provided in the above drawings. Therefore, it has been decided that there is no restriction in changing internal layout of the building while maintaining the front rear and side façade of the building in conformity with the Architectural Control Sheets/Standard Designs and Frame Controls. However, the internal changes should be strictly meet the provisions of HUDA (Erection of Buildings) Regulation, 1979. These instructions be strictly adhered to. Sd/- Chief Town Planner, HUDA, Panchkula Endst. NO CTP (H)/6801-6822 Dated: 29.12.2005 A copy is forwarded to the following for information and necessary action:- 1. Administrator, Panchkula, Gurgaon, Faridabad & Hisar. 2. Estate Officer, HUDA, Panchkula, Ambala, Gurgaon, Faridabad, Karnal, Kaithal, Kurukshetra, Panipat, Hissar, Rohtak, Bahadurgarh, Bhiwani, Jind Sirsa, Sonepat, Yamuna Nagar & Rewari. 3. Senior Architect, HUDA, Panchkula. Sd/- Chief Town Planner, HUDA, Panchkula . -

Haryana State Development Report
RYAN HA A Haryana Development Report PLANNING COMMISSION GOVERNMENT OF INDIA NEW DELHI Published by ACADEMIC FOUNDATION NEW DELHI First Published in 2009 by e l e c t Academic Foundation x 2 AF 4772-73 / 23 Bharat Ram Road, (23 Ansari Road), Darya Ganj, New Delhi - 110 002 (India). Phones : 23245001 / 02 / 03 / 04. Fax : +91-11-23245005. E-mail : [email protected] www.academicfoundation.com a o m Published under arrangement with : i t x 2 Planning Commission, Government of India, New Delhi. Copyright : Planning Commission, Government of India. Cover-design copyright : Academic Foundation, New Delhi. © 2009. ALL RIGHTS RESERVED. No part of this book shall be reproduced, stored in a retrieval system, or transmitted by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of, and acknowledgement of the publisher and the copyright holder. Cataloging in Publication Data--DK Courtesy: D.K. Agencies (P) Ltd. <[email protected]> Haryana development report / Planning Commission, Government of India. p. cm. Includes bibliographical references (p. ). ISBN 13: 9788171887132 ISBN 10: 8171887139 1. Haryana (India)--Economic conditions. 2. Haryana (India)--Economic policy. 3. Natural resources--India-- Haryana. I. India. Planning Commission. DDC 330.954 558 22 Designed and typeset by Italics India, New Delhi Printed and bound in India. LIST OF TABLES ARYAN 5 H A Core Committee (i) Dr. (Mrs.) Syeda Hameed Chairperson Member, Planning Commission, New Delhi (ii) Smt. Manjulika Gautam Member Senior Adviser (SP-N), Planning Commission, New Delhi (iii) Principal Secretary (Planning Department) Member Government of Haryana, Chandigarh (iv) Prof. Shri Bhagwan Dahiya Member (Co-opted) Director, Institute of Development Studies, Maharshi Dayanand University, Rohtak (v) Dr. -

Village & Townwise Primary Census Abstract, Rohtak, Part XII-A & B
CENSUS OF INDIA 1991 SERIES-8 HARYANA DISTRICT CENSUS HANDBOOK PART XII - A & B VILLAGE &TOWN DIRECTORY VILLAGE & TOWNWISE PRIMARY CENSUS ABSTRACT DISTRICT ROHTAfK v.s. CHAUDHRI DI rector of Census Operations Haryana Published by : The Government of Haryana, ]993 HARYANA DISTRICT ROHTAK Km5 0 5 10 15 10Km bLa:::EL__ :L I _ b -~ (Jl o DISTRICT ROHTAK CHANGE IN JURISDICTION 1981-91 z Km 10 0 10 Km L-.L__j o fTI r BOUNDARY, STATE! UNION TERRi10RY DIS TRICT TAHSIL AREA GAINED FROM DISTRIC1 SONIPAT AREA GAINED FROM DISTRICT HISAR \[? De/fl/ ..... AREA LOST TO DISTRICT SONIPAT AREA LOST TO NEWLY CREATED \ DISTRICT REWARI t-,· ~ .'7;URt[oN~ PART OF TAHSIL JHAJJAR OF ROHTAK FALLS IN C.D,BLO,CK DADRI-I . l...../'. ",evA DISTRICT BHIWANI \ ,CJ ~Q . , ~ I C.D.BLOCK BOUNDARY "- EXCLUDES STATUTORY ~:'"t -BOUNDARY, STATE I UNION TERRITORY TOWN (S) ',1 DISTRICT BOUNDARIES ARE UPDATED TAHSIL. UPTO I. 1.1990 C.D.BLOCK HEADQUARTERS '. DISTRICT; TAHSIL; C. D. BLOCK. NATIONAL HIGHWAY STATE HIGHWAY SH 20 C. O. BLOCKS IMPORTANT METALLED ROAD RS A MUNDlANA H SAMPLA RAILWAY LINE WITH STATION, BROAD GAUGE IiiiiI METRE GAUGE RS B GOHANA I BERI CANAL 111111""1111111 VILLAGE HAVING 5000 AND ABOVE POPULATION WITH NAME Jagsi C KATHURA J JHAJJAR URBAN AREA WITH POPULATION SIZE-CLASS I,!I,lII, IV & V" ••• ••• 0 LAKHAN MAJRA K MATENHAIL POST AND TELEGRAPH OFFICE PTO DEGREE COLLEGE AND TECHNICAL INSTITUTION .. [liiJ C'a E MAHAM L SAHLAWAS REST ClOUSE, TRAVELLERS' BUNGALOW' AND CANAL BUNGALOW RH, TB, CB F KALANAUR BAHADURGARH Other villages having PTO IRH/TBIC8 ~tc.,are shown as. -

Schedule for 2Nd Dose of Covishield Revised
WWW.YUGMARG.COM FOLLOW US ON REGD NO. CHD/0061/2006-08 | RNI NO. 61323/95 Join us at telegram https://t.me/yugmarg Tuesday March 23, 2021 CHANDIGARH, VOL. XXVI, NO. 53 PAGES 12, RS. 2 YOUR REGION, YOUR PAPER 6084 bottles of illicit Balbir Sidhu flags Himachal Chief Umpire's Call is liquor recovered, off Medicine Minister Jai Ram creating lot of honours 'Loktantra truck impounded Delivery Van confusion, grey Praharies' area needs to be addressed, says Kohli PAGE 3 PAGE 4 PAGE 5 PAGE 12 Schedule for 2nd dose Param Bir moves SC, seeks CBI of Covishield revised probe in alleged 'malpractices' Now, vaccine to be administered after 4-8 weeks AGENCY UTs on Monday, No tuition fee NEW DELHI, MAR 22 Union Health Secre- AGENCY tary Rajesh for PG girls NEW DELHI, MAR 22 Pawar defends Anil At a time when the coun- Bhushan said the in Haryana try is in the midst of the ministry has accepted Param Bir Singh, former Mumbai Police Deshmukh second phase of covid-19 the recommendations CHANDIGARH: Tuition Commissioner on Monday filed a plea be- vaccination, the Centre on of NTAGI and NEG- fees will not be charged fore the Supreme Court, seeking a direc- MUMBAI: Following former Com- Monday revised the time VAC. from girl students pursu- tion to the CBI to immediately conduct an missioner of Police of Mumbai gap between administra- Keeping the ex- ing post-graduation in unbiased, uninfluenced, impartial and fair Param Bir Singh's allegations of cor- tion of the two doses of isting scientific evi- Government and Gov- investigation in the various corrupt mal- ruption against Anil Deshmukh Serum Institute’s Cov- dence in view, it ap- ernment Aided Colleges practices of Maharashtra Home Minister causing a political storm in the state, ishield vaccine.