LIMITING FACTORS for Northern FLYING
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Breeding Ecology of Barred Owls in the Central Appalachians
BREEDING ECOLOGY OF BARRED OWLS IN THE CENTRAL APPALACHIANS ABSTRACT- Eight pairs of breedingBarred Owls (Strix varia) in westernMaryland were studied. Nest site habitat was sampledand quantifiedusing a modificationof theJames and Shugart(1970) technique (see Titus and Mosher1981). Statisticalcomparison to 76 randomhabitat plots showed nest sites werb in moremature forest stands and closer to forest openings.There was no apparent association of nestsites with water. Cavity dimensions were compared statistically with 41 randomlyselected cavities. Except for cavityheight, there were no statistically significant differences between them. Smallmammals comprised 65.9% of the totalnumber of prey itemsrecorded, of which81.5% were members of the familiesCricetidae and Soricidae. Birds accounted for 14.6%of theprey items and crayfish and insects 19.5%. We also recordedan apparentinstance of juvenile cannibalism. Thirteennestlings were produced in 7 nests,averaging 1.9 young per nest.Only 2 of 5 nests,where the outcome was known,fledged young. The Barred Owl (Strix varia) is a common noc- STUDY AREA AND METHODS turnal raptor in forestsof the easternUnited States, The studywas conducted in Green Ridge State Forest (GRSF), though few detailedstudies of it havebeen pub- Allegany County, Maryland. It is within the Ridge and Valley lished.Most reportsare of singlenesting occurr- physiographicregion (Stoneand Matthews 1977), characterized by narrowmountain ridges oriented northeast to southwestsepa- encesand general observations(Bolles 1890; Carter rated by steepnarrow valleys(see Titus 1980). 1925; Henderson 1933; Robertson 1959; Brown About 74% of the countyand nearly all of GRSF is forested 1962; Caldwell 1972; Hamerstrom1973; Appel- Major foresttypes were describedby Brushet al. (1980).Predom- gate 1975; Soucy 1976; Bird and Wright 1977; inant tree speciesinclude white oak (Quercusalba), red oak (Q. -

Phylogenies of Flying Squirrels (Pteromyinae)
Journal of Mammalian Evolution, Vol. 9, No. 1/2, June 2002 (© 2002) Phylogenies of Flying Squirrels (Pteromyinae) Richard W. Thorington, Jr.,'''* Diane Pitassy,^ and Sharon A. Jansa^ The phylogeny of flying squirrels was assessed, based on analyses of 80 morphological characters. Three published hypotheses were tested with constraint trees and compared with trees based on heuristic searches, all using PAUP*. Analyses were conducted on unordered data, on ordered data (Wagner), and on ordered data using Dollo parsimony. Compared with trees based on heuristic searches, the McKenna (1962) constraint trees were consistently the longsst, requiring 8-11 more steps. The Mein (1970) constraint trees were shorter, requiring five to seven steps more than the unconstrained trees, and the Thorington and Darrow (20(X)) constraint trees were shorter yet, zero to one step longer than the corresponding unconstrained tree. In each of the constraint trees, some of the constrained nodes had poor character support. The heuristic trees provided best character support for three groups, but they did not resolve the basal trichotomy between a Glaucomys group of six genera, a Petaurista group of four genera, and a Trogopterus group of four genera. The inclusion of the small northern Eurasian flying squirrel, Pteminys, in the Peiaiiiisia group of giant South Asian flying squirrels is an unexpected hypothesis. Another novel hypothesis is the inclusion of the genus Aemmys, large animals from the Sunda Shelf, with the Trogopterus group of smaller "complex-toothed flying squirrels" from mainland Malaysia and southeast Asia. We explore the implications of this study for future analysis of molecular data and for past and future interpretations of the fossil record. -

Phylogenetic Relationships Among Six Flying Squirrel Gener,A Inferred from Mitochondrial Cytochrome B Gene Sequences
Phylogenetic Relationships among Six Flying Squirrel Gener,a Inferred from Mitochondrial Cytochrome b Gene Sequences 著者(英) Oshida Tatsuo, Lin Liang-Kong, Yanagisawa Hisashi, Endo Hideki, Masuda Ryuichi journal or Zoological Science publication title volume 17 number 4 page range 485-489 year 2000-05 URL http://id.nii.ac.jp/1588/00004215/ ZOOLOGICAL SCIENCE 17: 485–489 (2000) © 2000 Zoological Society of Japan Phylogenetic Relationships among Six Flying Squirrel Genera, Inferred from Mitochondrial Cytochrome b Gene Sequences Tatsuo Oshida1*, Liang-Kong Lin2, Hisashi Yanagawa3, Hideki Endo4 and Ryuichi Masuda1 1Chromosome Research Unit, Faculty of Science, Hokkaido University, Sapporo 060-0810, Japan 2Laboratory of Wildlife Ecology, Department of Biology, Tunghai University, Taichung 407, Taiwan 3Laboratory of Wildlife Ecology, Obihiro University and Veterinary Medicine, Obihiro 080-0843, Japan 4Department of Zoology, National Science Museum, Shinjuku-ku, Tokyo, 169-0073, Japan ABSTRACT—Petauristinae (flying squirrels) consists of 44 extant species in 14 recent genera, and their phylogenetic relationships and taxonomy are unsettled questions. We analyzed partial mitochondrial cyto- chrome b gene sequences (1,068 base pairs) to investigate the phylogenetic relationships among six flying squirrel genera (Belomys, Hylopetes, Petaurista, Petinomys, and Pteromys from Asia and Glaucomys from North America). Molecular phylogenetic trees, constructed by neighbor-joining and maximum likelihood methods, strongly indicated the closer relationship between Hylopetes and Petinomys with 100% bootstrap values. Belomys early split from other flying squirrels. Petaurista was closely related to Pteromys, and Glaucomys was most closely related to the cluster consisting of Hylopetes and Petinomys. The bootstrap values supporting branching at the deeper nodes were not always so high, suggesting the early radiation in the evolution of flying squirrels. -

Nest Box Utility for Arboreal Small Mammals in Vietnam's Tropical Forest Использование Дуплянок Для И
Russian J. Theriol. 10(2): 5964 © RUSSIAN JOURNAL OF THERIOLOGY, 2011 Nest box utility for arboreal small mammals in Vietnams tropical forest Ami Kato, Tatsuo Oshida, Son Truong Nguyen, Nghia Xuan Nguyen, Hao Van Luon, Tue Van Ha, Bich Quang Truong, Hideki Endo & Dang Xuan Nguyen ABSTRACT. To test nest box utility in a Southeast Asian tropical forest, we set 30 wooden nest boxes on trees in the Cuc Phuong National Park, Vietnam for a year. During the rainy season, we checked each nest box each month in the daytime. We expected that arboreal rodents might be more likely to use the nest boxes as shelter from the heavy rain. During dry season, we additionally checked each nest box every two months. We expected that nest boxes would be used as a shelter from the rain by small arboreal mammals, such as rats and flying squirrels in the rainy season more than in the dry season. During the rainy season, we found ants, bees, and birds mainly nested the nest boxes for reproduction: bees in April; ants from May to August; and birds from April to June. From the late rainy season to the dry season, arboreal small mammals mainly used nest boxes: rats from August to February and flying squirrel in December. Nest resource competition between birds and rodents may be minimal since they use cavities in different seasons. Also, unlike our expectation, it was preliminary suggested that arboreal small rodents would use more frequently nest box in the dry season than in the rainy season. KEY WORDS: flying squirrel, rat, rainy season, dry season, competition, nest resource, nest material. -

Tree Squirrels: Managing Habitat and Controlling Damage
■ ,VVXHG LQ IXUWKHUDQFH RI WKH &RRSHUDWLYH ([WHQVLRQ :RUN$FWV RI 0D\ DQG -XQH LQ FRRSHUDWLRQ ZLWK WKH 8QLWHG 6WDWHV 'HSDUWPHQWRI$JULFXOWXUH 'LUHFWRU&RRSHUDWLYH([WHQVLRQ8QLYHUVLW\RI0LVVRXUL&ROXPELD02 NATURAL ■ ■ ■ DQHTXDORSSRUWXQLW\$'$LQVWLWXWLRQ H[WHQVLRQPLVVRXULHGX RESOURCES Tree Squirrels: Managing Habitat and Controlling Damage issouri is home to three species of tree squirrels: the fox squirrel (Sciurus niger) and gray squirrel M(S. carolinensis), both popular game animals; and the southern flying squirrel Glaucomys( volans), the smallest of the tree squirrel species in the state. These squirrels provide relaxation and enjoyment for many Missourians who spend time observing or photo- graphing wildlife. They seldom pose problems in rural areas, but it is not unusual for them to become a nuisance in urban areas (Figure 1). Occasionally, fox or gray squirrels enter attics and chimneys and cause damage to electrical wiring, siding or insulation. Squirrels may cause damage to home gardens and ornamentals; sweet corn, tomatoes and other vegetables or flower bulbs; and newly planted seeds in urban gardens. Squirrels also can become a nuisance around bird feeders, frightening birds and scattering seeds. Figure 1. Squirrels can become a nuisance, especially in urban areas. Fox squirrels Gray squirrels normally prefer areas with more forest Fox squirrels are most common in urban areas and cover than fox squirrels. Forests dominated by oaks and open woodlots. They are the largest of the three species, hickories are prime habitats for gray squirrels. Gray averaging 19 to 29 inches from nose to tail and weighing 1 squirrels also prefer to spend more time in trees and do not to 3 pounds. -

Northern Flying Squirrel (Glaucomys Sabrinus) Species Guidance Family: Sciuridae – the Squirrels
Northern Flying Squirrel (Glaucomys sabrinus) Species Guidance Family: Sciuridae – the squirrels Species of Greatest Conservation Need (SGCN) State Status: SC/P (Special Concern/Fully Protected) State Rank: S3S4 Federal Status: None Global Rank: G5 Wildlife Action Plan Counties with documented locations Mean Risk Score: 3.2 of northern flying squirrels in Wildlife Action Plan Area of Wisconsin. Source: Natural Heritage Photo by Ryan Stephens Importance Score: 2 Inventory Database, April 2013. Species Information General Description: The northern flying squirrel and its sister species, the southern flying squirrel (Glaucomys volans), both occur in Wisconsin. The northern flying squirrel is slightly larger than the southern flying squirrel, but is small compared to other tree squirrels. Adult northern flying squirrels in the Great Lakes region weigh 70-130 g (2.5-4.6 oz) (Kurta 1995). Total length (including tail) ranges from 245-315 mm (10-12 in), tail length 110-150 mm (4.3-5.9 in), hindfoot length 35-40 mm (1.4-1.6 in), and ear height 18-26 mm (0.7-1.0 in) (Jackson 1961, Kurta 1995). Pelage (fur) is silky and usually cinnamon–colored, but can range from dark brown to red. Belly hair is white at the tips and gray at the base. The northern flying squirrel uses its patagium (loose flap of skin between the front and hind legs; Fig. 1) for gliding between trees. A cartilaginous projection called a styliform process extends from the wrist (Fig. 1) to widen the patagium and enhance its effect (Wells-Gosling and Heaney 1989). Similar Species: The flying squirrels can easily be distinguished from the other eight Wisconsin squirrels by the presence of a furry patagium (flap of skin) that runs from the wrist to the ankle, and also by a cartilaginous projection on the wrist called a styliform process. -

FLYING SQUIRRELS Southern Flying Squirrel: Glaucomys Volans Northern Flying Squirrel: Glaucomys Sabrinus
WILDLIFE IN CONNECTICUT INFORMATIONAL SERIES FLYING SQUIRRELS Southern flying squirrel: Glaucomys volans Northern flying squirrel: Glaucomys sabrinus Habitat: Mature deciduous or mixed deciduous/ Length: Southern flying squirrel, 8 to 10 inches. coniferous forests with an abundance of various nut- Northern flying squirrel, 9.8 to 11.5 inches. producing trees. Food: Acorns, nuts, seeds, berries, blossoms, mush- Weight: Southern flying squirrel, 1.8 to 2.5 ounces. rooms, moths, beetles, and small birds and their eggs. Northern flying squirrel, 2 to 4.4 ounces. Identification: Flying squirrels have soft, gray-brown fur summer. The young are born blind and helpless but on the back and sides, with white underparts, a flattened develop more quickly than other squirrels. By six weeks tail and large, dark eyes for night vision. The northern of age, they are able to forage on their own. Tree flying squirrel is slightly darker and redder than the cavities and even bird houses may be used as nesting southern flying squirrel. The loose folds of skin between sites. The nest may be lined with shredded bark, leaves, the front and hind legs of these squirrels enables them moss, feathers, and other materials. to “fly;” they actually glide through the air on the History in Connecticut: The southern squirrel is found stretched surface of this loose skin. throughout Connecticut, but the northern flying squirrel's Range: The southern flying squirrel is found from range includes only the higher elevations of the north- southern Canada south to southern Florida, west to western part of the state. Due to their nocturnal nature, Minnesota and eastern Texas. -

SAN BERNARDINO FLYING SQUIRREL (Glaucomys Sabrinus Californicus) AS THREATENED OR ENDANGERED UNDER the UNITED STATES ENDANGERED SPECIES ACT
BEFORE THE SECRETARY OF INTERIOR PETITION TO LIST THE SAN BERNARDINO FLYING SQUIRREL (Glaucomys sabrinus californicus) AS THREATENED OR ENDANGERED UNDER THE UNITED STATES ENDANGERED SPECIES ACT Northern flying squirrel, Dr. Lloyd Glenn Ingles © California Academy of Sciences CENTER FOR BIOLOGICAL DIVERSITY, PETITIONER AUGUST 24, 2010 NOTICE OF PETITION Ken Salazar, Secretary of the Interior Ren Lohoefener, Regional Director U.S. Department of the Interior U.S. Fish and Wildlife Service Region 8 1849 C Street, N.W. 2800 Cottage Way, W-2606 Washington, DC 20240 Sacramento, CA 95825 Phone: (202) 208-3100 Phone: (503) 231-6118 [email protected] [email protected] Rowan Gould, Acting Director U.S. Fish and Wildlife Service 1849 C Street, NW, Mail Stop 3012 Washington, D.C. 20240 Phone: (202) 208-4717 Fax: (202) 208-6965 [email protected] PETITIONER Shaye Wolf, Ph.D. Center for Biological Diversity 351 California Street, Suite 600 San Francisco, CA 94104 office: (415) 632-5301 cell: (415) 385-5746 fax: (415) 436-9683 [email protected] __________________________ Date this 24th day of August, 2010 Pursuant to Section 4(b) of the Endangered Species Act (“ESA”), 16 U.S.C. §1533(b), Section 553(3) of the Administrative Procedures Act, 5 U.S.C. § 553(e), and 50 C.F.R. § 424.14(a), the Center for Biological Diversity hereby petitions the Secretary of the Interior, through the United States Fish and Wildlife Service (“USFWS”), to list the San Bernardino flying squirrel (Glaucomys sabrinus californicus) as a threatened or endangered species and to designate critical habitat to ensure its survival and recovery. -

Management and Conservation of Tree Squirrels: the Importance of Endemism, Species Richness, and Forest Condition
Management and Conservation of Tree Squirrels: The Importance of Endemism, Species Richness, and Forest Condition John L. Koprowski Wildlife and Fisheries Science, School of Natural Resources, University of Arizona, Tucson, AZ Abstract—Tree squirrels are excellent indicators of forest health yet the taxon is understudied. Most tree squirrels in the Holarctic Region are imperiled with some level of legal protection. The Madrean Archipelago is the epicenter for tree squirrel diversity in North America with 5 endemic species and 2 introduced species. Most species of the region are poorly studied in keeping with an international dearth of data on this taxon; 3 of the 5 native species are the subject of <3 publications. Herein, I review literature on the response of squirrels to forest management from clearcutting to less comprehensive operations. Major threats to squirrel diversity in the Madrean Archipelago’s Sky Islands are the introduction of species, altered fire regimes, and inappropriate application of forestry practices. of arboreal Sciuridae world-wide yielded a bleak picture with Introduction 13% of known species the subject of >1 publication. The Madrean Archipelago is renown for its biodiversity Five Sciurus and Tamiasciurus are native to the Sky Islands; (Lomolino et al. 1987). The area is considered a hotspot of 2 Sciurus were introduced (table 1), the greatest diversity in the evolution (Spector 2002) and contains the greatest diversity of Holarctic Region. When including 16 unique subspecies, the mammals in the United States (Turner et al. 1995). Despite this Madrean Archipelago’s diversity doubles that found elsewhere. megadiversity, the fauna is poorly represented in peer-reviewed Most forms diverged in isolation in the Madrean Archipelago scientific literature (Koprowski et al., this proceedings). -

Locomotion, Morphology, and Habitat Use in Arboreal
LOCOMOTION, MORPHOLOGY, AND HABITAT USE IN ARBOREAL SQUIRRELS (RODENTIA: SCIURIDAE) A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Richard L. Essner, Jr. June 2003 This dissertation entitled LOCOMOTION, MORPHOLOGY, AND HABITAT USE IN ARBOREAL SQUIRRELS (RODENTIA: SCIURIDAE) BY RICHARD L. ESSNER, JR. has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Stephen M. Reilly Associate Professor of Biological Sciences Leslie A. Flemming Dean, College of Arts and Sciences ESSNER, JR., RICHARD L. Ph.D. June 2003. Biological Sciences Locomotion, Morphology, and Habitat Use in Arboreal Squirrels (Rodentia: Sciuridae) (135pp.) Director of Dissertation: Stephen M. Reilly Arboreal locomotion has not been well studied in mammals outside of primates and mammalian gliding has received even less attention. While numerous studies have examined morphological variation in these forms, there is currently a lack of detailed kinematic, behavioral, and ecological data to assist in explaining the patterns. Here, I present three studies that focus on differing aspects of locomotion in arboreal squirrels. These range from 3-D kinematics (Chapters 1 & 2) to morphology, locomotor behavior, and habitat use (Chapter 3). First, kinematics were quantified and compared among leaping, parachuting, and gliding squirrels to test for differences during the launch phase. Only six out of 23 variables were found to differ significantly among the three species investigated. The six significant variables were partitioned into morphological, behavioral, and performance based differences. Remarkably, there were no differences attributable to hindlimb kinematics indicating that propulsion is the same in leaping, parachuting, and gliding squirrels. -

Brookings, South Dakota, USA, (SCP), Department of Biology, University of Scranton, Scranton, PA 18510 USA (GGK)
THE JOHNS HOPKINS UNIVERSITY PRESS MOUNTAIN GORILLAS ELEPHANTS AND MAMMALOGY BIOLOGY, CONSERVATION, AND ETHICS ADAPTATION, DIVERSITY, ECOLOGY COEXISTENCE TOWARD A MORALITY OF third edition Gene Eckhart and Annette Lanjouw COEXISTENCE George A. Feldhamer, “The survival of edited by Christen Wemmer and Lee C. Drickamer, Stephen H. Vessey, mountain goril- Catherine A. Christen Joseph F. Merritt, and Carey Krajewski las depends foreword by John Seidensticker “This attractive wholly on our “An important book will be knowledge, and timely welcome to interest, and contribution to those seeking compassion, the elephant a well-written, forever. This debate.” current text book, with its —Beth Stevens, to use in their vivid photo- Disney’s mammalogy graphs, brings Animal courses.” us up-to-date on the plight and the Kingdom —Journal of promise of mountain gorilla conser- and Animal Mammalogy vation.”—George Schaller, Wildlife Programs $99.50 hardcover Conservation Society $75.00 hardcover $34.95 hardcover THE RISE OF ANIMALS CHARLES DARWIN EVOLUTION AND DIVERSIFICATION THE SOCIAL BEHAVIOR THE CONCISE STORY OF AN OF THE KINGDOM ANIMALIA OF OLDER ANIMALS EXTRAORDINARY MAN Mikhail A. Fedonkin, James G. Gehling, Anne Innis Dagg Tim M. Berra Kathleen Grey, Guy M. Narbonne, and Patricia Vickers-Rich This theme-span- Published to foreword by Arthur C. Clarke ning study reveals coincide with the the complex 200th anniver- “It’s a beautiful nature of sary of Charles book and the maturity in scores Darwin’s birth, definitive ac- of social species this compact count of the pe- and shows that biography tells riod . I love animal behavior the fascinating it and expect often displays the story of the per- it to become a same diversity we son and the idea classic.” find in ourselves. -
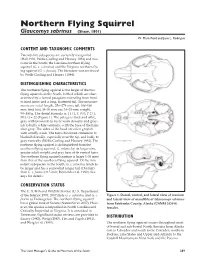
Northern Flying Squirrel, Galucomys Sabrinus
Glaucomys sabrinus (Shaw, 1801) NFSQ W. Mark Ford and Jane L. Rodrigue CONTENT AND TAXONOMIC COMMENTS Twenty-five subspecies are currently recognized (Hall 1981, Wells-Gosling and Heaney 1984) and two occur in the South: the Carolina northern flying squirrel (G. s. coloratus) and the Virginia northern fly- ing squirrel (G. s. fuscus). The literature was reviewed by Wells-Gosling and Heaney (1984). DISTINGUISHING CHARACTERISTICS The northern flying squirrel is the larger of the two flying squirrels in the South, both of which are char- acterized by a furred patagium extending from front to hind limbs and a long, flattened tail. The measure- ments are: total length, 256–274 mm; tail, 108–160 mm; hind foot, 34–39 mm; ear, 16–20 mm; weight, 90–140 g. The dental formula is: I 1/1, C 0/0, P 2/1, M 3/3 = 22 (Figure 1). The pelage is thick and silky, gray with brownish to rusty wash dorsally and gray- ish to buffy white ventrally, with the base of the hairs slategray.Thesidesoftheheadareoftengrayish with a buffy wash. The tail is bicolored cinnamon to blackish dorsally, especially near the tip, and buffy to gray ventrally (Wells-Gosling and Heaney 1984). The northern flying squirrel is distinguished from the southern flying squirrel, G. volans,byitslargersize, greater adult weight, and gray base of its ventral hairs. The northern flying squirrel cranium is larger (>36 mm) than that of the southern flying squirrel. Of the two extant subspecies in the South, G. s. coloratus tends to be larger and has a somewhat longer tail (134 mm) than G.