Supplementary Table 6
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Genetic Screening Identifies a Component of the SWI/SNF Complex, Arid1b As a Senescence Regulator
A genetic screening identifies a component of the SWI/SNF complex, Arid1b as a senescence regulator Sadaf Khan A thesis submitted to Imperial College London for the degree of Doctor in Philosophy MRC Clinical Sciences Centre Imperial College London, School of Medicine July 2013 Statement of originality All experiments included in this thesis were performed by myself unless otherwise stated. Copyright Declaration The copyright of this thesis rests with the author and is made available under a Creative Commons Attribution Non-Commercial No Derivatives license. Researchers are free to copy, distribute or transmit the thesis on the condition that they attribute it, that they do not use it for commercial purposes and that they do not alter, transform or build upon it. For any reuse or redistribution, researchers must make clear to others the license terms of this work. 2 Abstract Senescence is an important tumour suppressor mechanism, which prevents the proliferation of stressed or damaged cells. The use of RNA interference to identify genes with a role in senescence is an important tool in the discovery of novel cancer genes. In this work, a protocol was established for conducting bypass of senescence screenings, using shRNA libraries together with next-generation sequencing. Using this approach, the SWI/SNF subunit Arid1b was identified as a regulator of cellular lifespan in MEFs. SWI/SNF is a large multi-subunit complex that remodels chromatin. Mutations in SWI/SNF proteins are frequently associated with cancer, suggesting that SWI/SNF components are tumour suppressors. Here the role of ARID1B during senescence was investigated. Depletion of ARID1B extends the proliferative capacity of primary mouse and human fibroblasts. -

Identification of the Genes Up- and Down-Regulated by the High Mobility Group A1 (HMGA1) Proteins: Tissue Specificity of the HMGA1-Dependent Gene Regulation
[CANCER RESEARCH 64, 5728–5735, August 15, 2004] Identification of the Genes Up- and Down-Regulated by the High Mobility Group A1 (HMGA1) Proteins: Tissue Specificity of the HMGA1-Dependent Gene Regulation Josefina Martinez Hoyos,1 Monica Fedele,1 Sabrina Battista,1 Francesca Pentimalli,1,2 Mogens Kruhoffer,3 Claudio Arra,4 Torben F. Orntoft,3 Carlo Maria Croce,2 and Alfredo Fusco1 1Dipartimento di Biologia e Patologia Cellulare e Molecolare e/o Istituto di Endocrinologia ed Oncologia Sperimentale del CNR, Facolta` di Medicina e Chirurgia di Napoli, Universita` degli Studi di Napoli “Federico II,” Naples, Italy; 2Kimmel Cancer Center, Jefferson Medical College, Philadelphia, Pennsylvania; 3Department of Clinical Biochemistry, Aarhus University Hospital, Aarhus, Denmark; and 4Istituto Dei Tumori Di Napoli “Fondazione Pascale,” Naples, Italy. ABSTRACT To identify the differentiation pathways in which HMGA1 is in- volved and to assess the role of the HMGA1 proteins in development, High mobility group A (HMGA) proteins are chromatinic proteins that we generated embryonic stem (ES) cells in which one or both hmga1 do not have transcriptional activity per se, however, by interacting with alleles are disrupted. We reported recently that hmga1Ϫ/Ϫ ES cells the transcription machinery, they regulate, negatively or positively, the expression of several genes. We searched for genes regulated by HMGA1 generate less T-cell precursors than do wild-type ES cells after in proteins using microarray analysis in embryonic stem (ES) cells bearing vitro-specific differentiation. Indeed, they preferentially differentiate one or two disrupted hmga1 alleles. We identified 87 transcripts increased to B cells, probably consequent to decreased IL-2 expression and and 163 transcripts decreased of at least 4-fold in hmga1؊/؊ ES cells. -

Prioritization and Evaluation of Depression Candidate Genes by Combining Multidimensional Data Resources
Prioritization and Evaluation of Depression Candidate Genes by Combining Multidimensional Data Resources Chung-Feng Kao1, Yu-Sheng Fang2, Zhongming Zhao3, Po-Hsiu Kuo1,2,4* 1 Department of Public Health and Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei, Taiwan, 2 Institute of Clinical Medicine, School of Medicine, National Cheng-Kung University, Tainan, Taiwan, 3 Departments of Biomedical Informatics and Psychiatry, Vanderbilt University School of Medicine, Nashville, Tennessee, United States of America, 4 Research Center for Genes, Environment and Human Health, National Taiwan University, Taipei, Taiwan Abstract Background: Large scale and individual genetic studies have suggested numerous susceptible genes for depression in the past decade without conclusive results. There is a strong need to review and integrate multi-dimensional data for follow up validation. The present study aimed to apply prioritization procedures to build-up an evidence-based candidate genes dataset for depression. Methods: Depression candidate genes were collected in human and animal studies across various data resources. Each gene was scored according to its magnitude of evidence related to depression and was multiplied by a source-specific weight to form a combined score measure. All genes were evaluated through a prioritization system to obtain an optimal weight matrix to rank their relative importance with depression using the combined scores. The resulting candidate gene list for depression (DEPgenes) was further evaluated by a genome-wide association (GWA) dataset and microarray gene expression in human tissues. Results: A total of 5,055 candidate genes (4,850 genes from human and 387 genes from animal studies with 182 being overlapped) were included from seven data sources. -

Transcriptional Regulation of RKIP in Prostate Cancer Progression
Health Science Campus FINAL APPROVAL OF DISSERTATION Doctor of Philosophy in Biomedical Sciences Transcriptional Regulation of RKIP in Prostate Cancer Progression Submitted by: Sandra Marie Beach In partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biomedical Sciences Examination Committee Major Advisor: Kam Yeung, Ph.D. Academic William Maltese, Ph.D. Advisory Committee: Sonia Najjar, Ph.D. Han-Fei Ding, M.D., Ph.D. Manohar Ratnam, Ph.D. Senior Associate Dean College of Graduate Studies Michael S. Bisesi, Ph.D. Date of Defense: May 16, 2007 Transcriptional Regulation of RKIP in Prostate Cancer Progression Sandra Beach University of Toledo ACKNOWLDEGMENTS I thank my major advisor, Dr. Kam Yeung, for the opportunity to pursue my degree in his laboratory. I am also indebted to my advisory committee members past and present, Drs. Sonia Najjar, Han-Fei Ding, Manohar Ratnam, James Trempe, and Douglas Pittman for generously and judiciously guiding my studies and sharing reagents and equipment. I owe extended thanks to Dr. William Maltese as a committee member and chairman of my department for supporting my degree progress. The entire Department of Biochemistry and Cancer Biology has been most kind and helpful to me. Drs. Roy Collaco and Hong-Juan Cui have shared their excellent technical and practical advice with me throughout my studies. I thank members of the Yeung laboratory, Dr. Sungdae Park, Hui Hui Tang, Miranda Yeung for their support and collegiality. The data mining studies herein would not have been possible without the helpful advice of Dr. Robert Trumbly. I am also grateful for the exceptional assistance and shared microarray data of Dr. -

A Preliminary Transcriptome Analysis Suggests a Transitory Effect of Vitamin D on Mitochondrial Function in Obese Young Finnish Subjects
ID: 18-0537 8 5 E Einarsdottir et al. Effect of vitamin D on gene 8:5 559–570 expression RESEARCH A preliminary transcriptome analysis suggests a transitory effect of vitamin D on mitochondrial function in obese young Finnish subjects Elisabet Einarsdottir1,2,3,†, Minna Pekkinen1,4, Kaarel Krjutškov2,5, Shintaro Katayama3, Juha Kere1,2,3,6, Outi Mäkitie1,4,7,8 and Heli Viljakainen1,9 1Folkhälsan Institute of Genetics, University of Helsinki, Helsinki, Finland 2Molecular Neurology Research Program, University of Helsinki, Helsinki, Finland 3Department of Biosciences and Nutrition, Karolinska Institutet, Huddinge, Sweden 4Children’s Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland 5Competence Centre on Health Technologies, Tartu, Estonia 6School of Basic and Medical Biosciences, King’s College London, Guy’s Hospital, London, United Kingdom 7Department of Molecular Medicine and Surgery and Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden 8Department of Clinical Genetics, Karolinska University Hospital, Stockholm, Sweden 9Department of Food and Environmental Sciences, University of Helsinki, Helsinki, Finland Correspondence should be addressed to H Viljakainen: [email protected] †(E Einarsdottir is now at Department of Gene Technology, Science for Life Laboratory, KTH-Royal Institute of Technology, Solna, Sweden) Abstract Objective: The effect of vitamin D at the transcriptome level is poorly understood, Key Words and furthermore, it is unclear if it differs between obese and normal-weight subjects. f vitamin D The objective of the study was to explore the transcriptome effects of vitamin D f gene expression supplementation. f obesity Design and methods: We analysed peripheral blood gene expression using GlobinLock f transcriptome oligonucleotides followed by RNA sequencing in individuals participating in a 12-week f mitochondrial function randomised double-blinded placebo-controlled vitamin D intervention study. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Bppart and Bpmax: RNA-RNA Interaction Partition Function and Structure Prediction for the Base Pair Counting Model
BPPart and BPMax: RNA-RNA Interaction Partition Function and Structure Prediction for the Base Pair Counting Model Ali Ebrahimpour-Boroojeny, Sanjay Rajopadhye, and Hamidreza Chitsaz ∗ Department of Computer Science, Colorado State University Abstract A few elite classes of RNA-RNA interaction (RRI), with complex roles in cellular functions such as miRNA-target and lncRNAs in human health, have already been studied. Accordingly, RRI bioinfor- matics tools tailored for those elite classes have been proposed in the last decade. Interestingly, there are somewhat unnoticed mRNA-mRNA interactions in the literature with potentially drastic biological roles. Hence, there is a need for high-throughput generic RRI bioinformatics tools. We revisit our RRI partition function algorithm, piRNA, which happens to be the most comprehensive and computationally-intensive thermodynamic model for RRI. We propose simpler models that are shown to retain the vast majority of the thermodynamic information that piRNA captures. We simplify the energy model and instead consider only weighted base pair counting to obtain BPPart for Base-pair Partition function and BPMax for Base-pair Maximization which are 225 and 1350 faster ◦ × × than piRNA, with a correlation of 0.855 and 0.836 with piRNA at 37 C on 50,500 experimentally charac- terized RRIs. This correlation increases to 0.920 and 0.904, respectively, at 180◦C. − Finally, we apply our algorithm BPPart to discover two disease-related RNAs, SNORD3D and TRAF3, and hypothesize their potential roles in Parkinson's disease and Cerebral Autosomal Dominant Arteri- opathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL). 1 Introduction Since mid 1990s with the advent of RNA interference discovery, RNA-RNA interaction (RRI) has moved to the spotlight in modern, post-genome biology. -
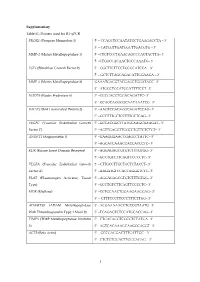
1 Supplementary Table S1. Primers Used for RT-Qpcr PROX1
Supplementary Table S1. Primers used for RT-qPCR PROX1 (Prospero Homeobox 1) 5’ – CCAGCTCCAATATGCTGAAGACCTA – 3’ 5’ – CATCGTTGATGGCTTGACGTG – 3‘ MMP-1 (Matrix Metallopeptidase 1) 5' –CTGTCCCTGAACAGCCCAGTACTTA– 3' 5' –CTGGCCACAACTGCCAAATG– 3' FGF2 (Fibroblast Growth Factor 2) 5′ - GGCTTCTTCCTGCGCATCCA – 3′ 5′ – GCTCTTAGCAGACATTGGAAGA – 3′ MMP-3 (Matrix Metallopeptidase 3) GAAATGAGGTACGAGCTGGATACC– 3’ 5’ –ATGGCTGCATCGATTTTCCT– 3’ NUDT6 (Nudix Hydrolase 6) 5’ –GGCGAGCTGGACAGATTC– 3’ 5’ –GCAGCAGGGGCAATAAATCG– 3’ BAIAP2 (BAI1 Associated Protein 2) 5’ –AAGTCCACAGGCAGATCCAG– 3’ 5’ –GCCTTTGCTCCTTTGCTCAG– 3’ VEGFC (Vascular Endothelial Growth 5’ –GCCACGGCTTATGCAAGCAAAGAT– 3’ Factor C) 5’ –AGTTGAGGTTGGCCTGTTCTCTGT– 3’ ANGPT1 (Angiopoietin 1) 5’ –GAAGGGAACCGAGCCTATTC– 3’ 5’ –AGCATCAAACCACCATCCTC– 3’ KDR (Kinase Insert Domain Receptor) 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ VEGFA (Vascular Endothelial Growth 5’ –CTTGCCTTGCTGCTCTACCT– 3’ Factor A) 5’ –AAGATGTCCACCAGGGTCTC– 3’ PLAT (Plasminogen Activator, Tissue 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ Type) 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ MDK (Midkine) 5’ –CCTGCAACTGGAAGAAGGAG– 3’ 5’ -- CTTTCCCTTCCCTTTCTTGG– 3’ ADAMTS9 (ADAM Metallopeptidase 5’ –ACGAAAAACCTGCCGTAATG– 3’ With Thrombospondin Type 1 Motif 9) 5’ –TCAGAGTCTCCATGCACCAG– 3’ TIMP3 (TIMP Metallopeptidase Inhibitor 5’ –CTGACAGGTCGCGTCTATGA– 3’ 3) 5’ –AGTCACAAAGCAAGGCAGGT– 3’ ACTB (Beta Actin) 5’ – GCCGAGGACTTTGATTGC – 3’ 5’– CTGTGTGGACTTGGGAGAG – 3’ 1 Figure S1. Efficient silencing of PROX1 in CGTH-W-1 and FTC-133 cells. Western blotting analysis shows a decrease in PROX1 protein level by targeting with siRNAs purchased from Santa Cruz (SC) and Sigma-Aldrich (SA) in both CGTH-W-1 and FTC-133 cell line. Beta-actin was used as a loading control of protein lysates. Figure S2. The tube formation assay. The silencing of PROX1 in CGTH-W-1 and FTC-133 cells enhances the angiogenesis in vitro of endothelial cells. HUVECs were cultured in 96-well plates coated with a semi-solid Matrigel. -

Supplemental Materials ZNF281 Enhances Cardiac Reprogramming
Supplemental Materials ZNF281 enhances cardiac reprogramming by modulating cardiac and inflammatory gene expression Huanyu Zhou, Maria Gabriela Morales, Hisayuki Hashimoto, Matthew E. Dickson, Kunhua Song, Wenduo Ye, Min S. Kim, Hanspeter Niederstrasser, Zhaoning Wang, Beibei Chen, Bruce A. Posner, Rhonda Bassel-Duby and Eric N. Olson Supplemental Table 1; related to Figure 1. Supplemental Table 2; related to Figure 1. Supplemental Table 3; related to the “quantitative mRNA measurement” in Materials and Methods section. Supplemental Table 4; related to the “ChIP-seq, gene ontology and pathway analysis” and “RNA-seq” and gene ontology analysis” in Materials and Methods section. Supplemental Figure S1; related to Figure 1. Supplemental Figure S2; related to Figure 2. Supplemental Figure S3; related to Figure 3. Supplemental Figure S4; related to Figure 4. Supplemental Figure S5; related to Figure 6. Supplemental Table S1. Genes included in human retroviral ORF cDNA library. Gene Gene Gene Gene Gene Gene Gene Gene Symbol Symbol Symbol Symbol Symbol Symbol Symbol Symbol AATF BMP8A CEBPE CTNNB1 ESR2 GDF3 HOXA5 IL17D ADIPOQ BRPF1 CEBPG CUX1 ESRRA GDF6 HOXA6 IL17F ADNP BRPF3 CERS1 CX3CL1 ETS1 GIN1 HOXA7 IL18 AEBP1 BUD31 CERS2 CXCL10 ETS2 GLIS3 HOXB1 IL19 AFF4 C17ORF77 CERS4 CXCL11 ETV3 GMEB1 HOXB13 IL1A AHR C1QTNF4 CFL2 CXCL12 ETV7 GPBP1 HOXB5 IL1B AIMP1 C21ORF66 CHIA CXCL13 FAM3B GPER HOXB6 IL1F3 ALS2CR8 CBFA2T2 CIR1 CXCL14 FAM3D GPI HOXB7 IL1F5 ALX1 CBFA2T3 CITED1 CXCL16 FASLG GREM1 HOXB9 IL1F6 ARGFX CBFB CITED2 CXCL3 FBLN1 GREM2 HOXC4 IL1F7 -

1 AGING Supplementary Table 2
SUPPLEMENTARY TABLES Supplementary Table 1. Details of the eight domain chains of KIAA0101. Serial IDENTITY MAX IN COMP- INTERFACE ID POSITION RESOLUTION EXPERIMENT TYPE number START STOP SCORE IDENTITY LEX WITH CAVITY A 4D2G_D 52 - 69 52 69 100 100 2.65 Å PCNA X-RAY DIFFRACTION √ B 4D2G_E 52 - 69 52 69 100 100 2.65 Å PCNA X-RAY DIFFRACTION √ C 6EHT_D 52 - 71 52 71 100 100 3.2Å PCNA X-RAY DIFFRACTION √ D 6EHT_E 52 - 71 52 71 100 100 3.2Å PCNA X-RAY DIFFRACTION √ E 6GWS_D 41-72 41 72 100 100 3.2Å PCNA X-RAY DIFFRACTION √ F 6GWS_E 41-72 41 72 100 100 2.9Å PCNA X-RAY DIFFRACTION √ G 6GWS_F 41-72 41 72 100 100 2.9Å PCNA X-RAY DIFFRACTION √ H 6IIW_B 2-11 2 11 100 100 1.699Å UHRF1 X-RAY DIFFRACTION √ www.aging-us.com 1 AGING Supplementary Table 2. Significantly enriched gene ontology (GO) annotations (cellular components) of KIAA0101 in lung adenocarcinoma (LinkedOmics). Leading Description FDR Leading Edge Gene EdgeNum RAD51, SPC25, CCNB1, BIRC5, NCAPG, ZWINT, MAD2L1, SKA3, NUF2, BUB1B, CENPA, SKA1, AURKB, NEK2, CENPW, HJURP, NDC80, CDCA5, NCAPH, BUB1, ZWILCH, CENPK, KIF2C, AURKA, CENPN, TOP2A, CENPM, PLK1, ERCC6L, CDT1, CHEK1, SPAG5, CENPH, condensed 66 0 SPC24, NUP37, BLM, CENPE, BUB3, CDK2, FANCD2, CENPO, CENPF, BRCA1, DSN1, chromosome MKI67, NCAPG2, H2AFX, HMGB2, SUV39H1, CBX3, TUBG1, KNTC1, PPP1CC, SMC2, BANF1, NCAPD2, SKA2, NUP107, BRCA2, NUP85, ITGB3BP, SYCE2, TOPBP1, DMC1, SMC4, INCENP. RAD51, OIP5, CDK1, SPC25, CCNB1, BIRC5, NCAPG, ZWINT, MAD2L1, SKA3, NUF2, BUB1B, CENPA, SKA1, AURKB, NEK2, ESCO2, CENPW, HJURP, TTK, NDC80, CDCA5, BUB1, ZWILCH, CENPK, KIF2C, AURKA, DSCC1, CENPN, CDCA8, CENPM, PLK1, MCM6, ERCC6L, CDT1, HELLS, CHEK1, SPAG5, CENPH, PCNA, SPC24, CENPI, NUP37, FEN1, chromosomal 94 0 CENPL, BLM, KIF18A, CENPE, MCM4, BUB3, SUV39H2, MCM2, CDK2, PIF1, DNA2, region CENPO, CENPF, CHEK2, DSN1, H2AFX, MCM7, SUV39H1, MTBP, CBX3, RECQL4, KNTC1, PPP1CC, CENPP, CENPQ, PTGES3, NCAPD2, DYNLL1, SKA2, HAT1, NUP107, MCM5, MCM3, MSH2, BRCA2, NUP85, SSB, ITGB3BP, DMC1, INCENP, THOC3, XPO1, APEX1, XRCC5, KIF22, DCLRE1A, SEH1L, XRCC3, NSMCE2, RAD21. -

Identification of Novel Chemotherapeutic Strategies For
www.nature.com/scientificreports OPEN Identification of novel chemotherapeutic strategies for metastatic uveal melanoma Received: 17 November 2016 Paolo Fagone1, Rosario Caltabiano2, Andrea Russo3, Gabriella Lupo1, Accepted: 09 February 2017 Carmelina Daniela Anfuso1, Maria Sofia Basile1, Antonio Longo3, Ferdinando Nicoletti1, Published: 17 March 2017 Rocco De Pasquale4, Massimo Libra1 & Michele Reibaldi3 Melanoma of the uveal tract accounts for approximately 5% of all melanomas and represents the most common primary intraocular malignancy. Despite improvements in diagnosis and more effective local therapies for primary cancer, the rate of metastatic death has not changed in the past forty years. In the present study, we made use of bioinformatics to analyze the data obtained from three public available microarray datasets on uveal melanoma in an attempt to identify novel putative chemotherapeutic options for the liver metastatic disease. We have first carried out a meta-analysis of publicly available whole-genome datasets, that included data from 132 patients, comparing metastatic vs. non metastatic uveal melanomas, in order to identify the most relevant genes characterizing the spreading of tumor to the liver. Subsequently, the L1000CDS2 web-based utility was used to predict small molecules and drugs targeting the metastatic uveal melanoma gene signature. The most promising drugs were found to be Cinnarizine, an anti-histaminic drug used for motion sickness, Digitoxigenin, a precursor of cardiac glycosides, and Clofazimine, a fat-soluble iminophenazine used in leprosy. In vitro and in vivo validation studies will be needed to confirm the efficacy of these molecules for the prevention and treatment of metastatic uveal melanoma. Uveal melanoma is the most common primary intraocular cancer, and after the skin, the uveal tract is the second most common location for melanoma1. -

Identification of Potential Key Genes and Pathway Linked with Sporadic Creutzfeldt-Jakob Disease Based on Integrated Bioinformatics Analyses
medRxiv preprint doi: https://doi.org/10.1101/2020.12.21.20248688; this version posted December 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. Identification of potential key genes and pathway linked with sporadic Creutzfeldt-Jakob disease based on integrated bioinformatics analyses Basavaraj Vastrad1, Chanabasayya Vastrad*2 , Iranna Kotturshetti 1. Department of Biochemistry, Basaveshwar College of Pharmacy, Gadag, Karnataka 582103, India. 2. Biostatistics and Bioinformatics, Chanabasava Nilaya, Bharthinagar, Dharwad 580001, Karanataka, India. 3. Department of Ayurveda, Rajiv Gandhi Education Society`s Ayurvedic Medical College, Ron, Karnataka 562209, India. * Chanabasayya Vastrad [email protected] Ph: +919480073398 Chanabasava Nilaya, Bharthinagar, Dharwad 580001 , Karanataka, India NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/2020.12.21.20248688; this version posted December 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. Abstract Sporadic Creutzfeldt-Jakob disease (sCJD) is neurodegenerative disease also called prion disease linked with poor prognosis. The aim of the current study was to illuminate the underlying molecular mechanisms of sCJD. The mRNA microarray dataset GSE124571 was downloaded from the Gene Expression Omnibus database. Differentially expressed genes (DEGs) were screened.