Comparison of Mirna Profiling During Airway Epithelial Repair in Undifferentiated And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Genetic Screening Identifies a Component of the SWI/SNF Complex, Arid1b As a Senescence Regulator
A genetic screening identifies a component of the SWI/SNF complex, Arid1b as a senescence regulator Sadaf Khan A thesis submitted to Imperial College London for the degree of Doctor in Philosophy MRC Clinical Sciences Centre Imperial College London, School of Medicine July 2013 Statement of originality All experiments included in this thesis were performed by myself unless otherwise stated. Copyright Declaration The copyright of this thesis rests with the author and is made available under a Creative Commons Attribution Non-Commercial No Derivatives license. Researchers are free to copy, distribute or transmit the thesis on the condition that they attribute it, that they do not use it for commercial purposes and that they do not alter, transform or build upon it. For any reuse or redistribution, researchers must make clear to others the license terms of this work. 2 Abstract Senescence is an important tumour suppressor mechanism, which prevents the proliferation of stressed or damaged cells. The use of RNA interference to identify genes with a role in senescence is an important tool in the discovery of novel cancer genes. In this work, a protocol was established for conducting bypass of senescence screenings, using shRNA libraries together with next-generation sequencing. Using this approach, the SWI/SNF subunit Arid1b was identified as a regulator of cellular lifespan in MEFs. SWI/SNF is a large multi-subunit complex that remodels chromatin. Mutations in SWI/SNF proteins are frequently associated with cancer, suggesting that SWI/SNF components are tumour suppressors. Here the role of ARID1B during senescence was investigated. Depletion of ARID1B extends the proliferative capacity of primary mouse and human fibroblasts. -

Identification of the Genes Up- and Down-Regulated by the High Mobility Group A1 (HMGA1) Proteins: Tissue Specificity of the HMGA1-Dependent Gene Regulation
[CANCER RESEARCH 64, 5728–5735, August 15, 2004] Identification of the Genes Up- and Down-Regulated by the High Mobility Group A1 (HMGA1) Proteins: Tissue Specificity of the HMGA1-Dependent Gene Regulation Josefina Martinez Hoyos,1 Monica Fedele,1 Sabrina Battista,1 Francesca Pentimalli,1,2 Mogens Kruhoffer,3 Claudio Arra,4 Torben F. Orntoft,3 Carlo Maria Croce,2 and Alfredo Fusco1 1Dipartimento di Biologia e Patologia Cellulare e Molecolare e/o Istituto di Endocrinologia ed Oncologia Sperimentale del CNR, Facolta` di Medicina e Chirurgia di Napoli, Universita` degli Studi di Napoli “Federico II,” Naples, Italy; 2Kimmel Cancer Center, Jefferson Medical College, Philadelphia, Pennsylvania; 3Department of Clinical Biochemistry, Aarhus University Hospital, Aarhus, Denmark; and 4Istituto Dei Tumori Di Napoli “Fondazione Pascale,” Naples, Italy. ABSTRACT To identify the differentiation pathways in which HMGA1 is in- volved and to assess the role of the HMGA1 proteins in development, High mobility group A (HMGA) proteins are chromatinic proteins that we generated embryonic stem (ES) cells in which one or both hmga1 do not have transcriptional activity per se, however, by interacting with alleles are disrupted. We reported recently that hmga1Ϫ/Ϫ ES cells the transcription machinery, they regulate, negatively or positively, the expression of several genes. We searched for genes regulated by HMGA1 generate less T-cell precursors than do wild-type ES cells after in proteins using microarray analysis in embryonic stem (ES) cells bearing vitro-specific differentiation. Indeed, they preferentially differentiate one or two disrupted hmga1 alleles. We identified 87 transcripts increased to B cells, probably consequent to decreased IL-2 expression and and 163 transcripts decreased of at least 4-fold in hmga1؊/؊ ES cells. -

A Preliminary Transcriptome Analysis Suggests a Transitory Effect of Vitamin D on Mitochondrial Function in Obese Young Finnish Subjects
ID: 18-0537 8 5 E Einarsdottir et al. Effect of vitamin D on gene 8:5 559–570 expression RESEARCH A preliminary transcriptome analysis suggests a transitory effect of vitamin D on mitochondrial function in obese young Finnish subjects Elisabet Einarsdottir1,2,3,†, Minna Pekkinen1,4, Kaarel Krjutškov2,5, Shintaro Katayama3, Juha Kere1,2,3,6, Outi Mäkitie1,4,7,8 and Heli Viljakainen1,9 1Folkhälsan Institute of Genetics, University of Helsinki, Helsinki, Finland 2Molecular Neurology Research Program, University of Helsinki, Helsinki, Finland 3Department of Biosciences and Nutrition, Karolinska Institutet, Huddinge, Sweden 4Children’s Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland 5Competence Centre on Health Technologies, Tartu, Estonia 6School of Basic and Medical Biosciences, King’s College London, Guy’s Hospital, London, United Kingdom 7Department of Molecular Medicine and Surgery and Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden 8Department of Clinical Genetics, Karolinska University Hospital, Stockholm, Sweden 9Department of Food and Environmental Sciences, University of Helsinki, Helsinki, Finland Correspondence should be addressed to H Viljakainen: [email protected] †(E Einarsdottir is now at Department of Gene Technology, Science for Life Laboratory, KTH-Royal Institute of Technology, Solna, Sweden) Abstract Objective: The effect of vitamin D at the transcriptome level is poorly understood, Key Words and furthermore, it is unclear if it differs between obese and normal-weight subjects. f vitamin D The objective of the study was to explore the transcriptome effects of vitamin D f gene expression supplementation. f obesity Design and methods: We analysed peripheral blood gene expression using GlobinLock f transcriptome oligonucleotides followed by RNA sequencing in individuals participating in a 12-week f mitochondrial function randomised double-blinded placebo-controlled vitamin D intervention study. -
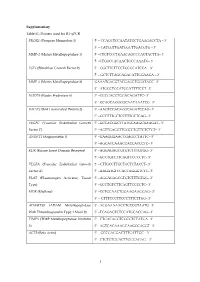
1 Supplementary Table S1. Primers Used for RT-Qpcr PROX1
Supplementary Table S1. Primers used for RT-qPCR PROX1 (Prospero Homeobox 1) 5’ – CCAGCTCCAATATGCTGAAGACCTA – 3’ 5’ – CATCGTTGATGGCTTGACGTG – 3‘ MMP-1 (Matrix Metallopeptidase 1) 5' –CTGTCCCTGAACAGCCCAGTACTTA– 3' 5' –CTGGCCACAACTGCCAAATG– 3' FGF2 (Fibroblast Growth Factor 2) 5′ - GGCTTCTTCCTGCGCATCCA – 3′ 5′ – GCTCTTAGCAGACATTGGAAGA – 3′ MMP-3 (Matrix Metallopeptidase 3) GAAATGAGGTACGAGCTGGATACC– 3’ 5’ –ATGGCTGCATCGATTTTCCT– 3’ NUDT6 (Nudix Hydrolase 6) 5’ –GGCGAGCTGGACAGATTC– 3’ 5’ –GCAGCAGGGGCAATAAATCG– 3’ BAIAP2 (BAI1 Associated Protein 2) 5’ –AAGTCCACAGGCAGATCCAG– 3’ 5’ –GCCTTTGCTCCTTTGCTCAG– 3’ VEGFC (Vascular Endothelial Growth 5’ –GCCACGGCTTATGCAAGCAAAGAT– 3’ Factor C) 5’ –AGTTGAGGTTGGCCTGTTCTCTGT– 3’ ANGPT1 (Angiopoietin 1) 5’ –GAAGGGAACCGAGCCTATTC– 3’ 5’ –AGCATCAAACCACCATCCTC– 3’ KDR (Kinase Insert Domain Receptor) 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ VEGFA (Vascular Endothelial Growth 5’ –CTTGCCTTGCTGCTCTACCT– 3’ Factor A) 5’ –AAGATGTCCACCAGGGTCTC– 3’ PLAT (Plasminogen Activator, Tissue 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ Type) 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ MDK (Midkine) 5’ –CCTGCAACTGGAAGAAGGAG– 3’ 5’ -- CTTTCCCTTCCCTTTCTTGG– 3’ ADAMTS9 (ADAM Metallopeptidase 5’ –ACGAAAAACCTGCCGTAATG– 3’ With Thrombospondin Type 1 Motif 9) 5’ –TCAGAGTCTCCATGCACCAG– 3’ TIMP3 (TIMP Metallopeptidase Inhibitor 5’ –CTGACAGGTCGCGTCTATGA– 3’ 3) 5’ –AGTCACAAAGCAAGGCAGGT– 3’ ACTB (Beta Actin) 5’ – GCCGAGGACTTTGATTGC – 3’ 5’– CTGTGTGGACTTGGGAGAG – 3’ 1 Figure S1. Efficient silencing of PROX1 in CGTH-W-1 and FTC-133 cells. Western blotting analysis shows a decrease in PROX1 protein level by targeting with siRNAs purchased from Santa Cruz (SC) and Sigma-Aldrich (SA) in both CGTH-W-1 and FTC-133 cell line. Beta-actin was used as a loading control of protein lysates. Figure S2. The tube formation assay. The silencing of PROX1 in CGTH-W-1 and FTC-133 cells enhances the angiogenesis in vitro of endothelial cells. HUVECs were cultured in 96-well plates coated with a semi-solid Matrigel. -

Supplemental Materials ZNF281 Enhances Cardiac Reprogramming
Supplemental Materials ZNF281 enhances cardiac reprogramming by modulating cardiac and inflammatory gene expression Huanyu Zhou, Maria Gabriela Morales, Hisayuki Hashimoto, Matthew E. Dickson, Kunhua Song, Wenduo Ye, Min S. Kim, Hanspeter Niederstrasser, Zhaoning Wang, Beibei Chen, Bruce A. Posner, Rhonda Bassel-Duby and Eric N. Olson Supplemental Table 1; related to Figure 1. Supplemental Table 2; related to Figure 1. Supplemental Table 3; related to the “quantitative mRNA measurement” in Materials and Methods section. Supplemental Table 4; related to the “ChIP-seq, gene ontology and pathway analysis” and “RNA-seq” and gene ontology analysis” in Materials and Methods section. Supplemental Figure S1; related to Figure 1. Supplemental Figure S2; related to Figure 2. Supplemental Figure S3; related to Figure 3. Supplemental Figure S4; related to Figure 4. Supplemental Figure S5; related to Figure 6. Supplemental Table S1. Genes included in human retroviral ORF cDNA library. Gene Gene Gene Gene Gene Gene Gene Gene Symbol Symbol Symbol Symbol Symbol Symbol Symbol Symbol AATF BMP8A CEBPE CTNNB1 ESR2 GDF3 HOXA5 IL17D ADIPOQ BRPF1 CEBPG CUX1 ESRRA GDF6 HOXA6 IL17F ADNP BRPF3 CERS1 CX3CL1 ETS1 GIN1 HOXA7 IL18 AEBP1 BUD31 CERS2 CXCL10 ETS2 GLIS3 HOXB1 IL19 AFF4 C17ORF77 CERS4 CXCL11 ETV3 GMEB1 HOXB13 IL1A AHR C1QTNF4 CFL2 CXCL12 ETV7 GPBP1 HOXB5 IL1B AIMP1 C21ORF66 CHIA CXCL13 FAM3B GPER HOXB6 IL1F3 ALS2CR8 CBFA2T2 CIR1 CXCL14 FAM3D GPI HOXB7 IL1F5 ALX1 CBFA2T3 CITED1 CXCL16 FASLG GREM1 HOXB9 IL1F6 ARGFX CBFB CITED2 CXCL3 FBLN1 GREM2 HOXC4 IL1F7 -

1 AGING Supplementary Table 2
SUPPLEMENTARY TABLES Supplementary Table 1. Details of the eight domain chains of KIAA0101. Serial IDENTITY MAX IN COMP- INTERFACE ID POSITION RESOLUTION EXPERIMENT TYPE number START STOP SCORE IDENTITY LEX WITH CAVITY A 4D2G_D 52 - 69 52 69 100 100 2.65 Å PCNA X-RAY DIFFRACTION √ B 4D2G_E 52 - 69 52 69 100 100 2.65 Å PCNA X-RAY DIFFRACTION √ C 6EHT_D 52 - 71 52 71 100 100 3.2Å PCNA X-RAY DIFFRACTION √ D 6EHT_E 52 - 71 52 71 100 100 3.2Å PCNA X-RAY DIFFRACTION √ E 6GWS_D 41-72 41 72 100 100 3.2Å PCNA X-RAY DIFFRACTION √ F 6GWS_E 41-72 41 72 100 100 2.9Å PCNA X-RAY DIFFRACTION √ G 6GWS_F 41-72 41 72 100 100 2.9Å PCNA X-RAY DIFFRACTION √ H 6IIW_B 2-11 2 11 100 100 1.699Å UHRF1 X-RAY DIFFRACTION √ www.aging-us.com 1 AGING Supplementary Table 2. Significantly enriched gene ontology (GO) annotations (cellular components) of KIAA0101 in lung adenocarcinoma (LinkedOmics). Leading Description FDR Leading Edge Gene EdgeNum RAD51, SPC25, CCNB1, BIRC5, NCAPG, ZWINT, MAD2L1, SKA3, NUF2, BUB1B, CENPA, SKA1, AURKB, NEK2, CENPW, HJURP, NDC80, CDCA5, NCAPH, BUB1, ZWILCH, CENPK, KIF2C, AURKA, CENPN, TOP2A, CENPM, PLK1, ERCC6L, CDT1, CHEK1, SPAG5, CENPH, condensed 66 0 SPC24, NUP37, BLM, CENPE, BUB3, CDK2, FANCD2, CENPO, CENPF, BRCA1, DSN1, chromosome MKI67, NCAPG2, H2AFX, HMGB2, SUV39H1, CBX3, TUBG1, KNTC1, PPP1CC, SMC2, BANF1, NCAPD2, SKA2, NUP107, BRCA2, NUP85, ITGB3BP, SYCE2, TOPBP1, DMC1, SMC4, INCENP. RAD51, OIP5, CDK1, SPC25, CCNB1, BIRC5, NCAPG, ZWINT, MAD2L1, SKA3, NUF2, BUB1B, CENPA, SKA1, AURKB, NEK2, ESCO2, CENPW, HJURP, TTK, NDC80, CDCA5, BUB1, ZWILCH, CENPK, KIF2C, AURKA, DSCC1, CENPN, CDCA8, CENPM, PLK1, MCM6, ERCC6L, CDT1, HELLS, CHEK1, SPAG5, CENPH, PCNA, SPC24, CENPI, NUP37, FEN1, chromosomal 94 0 CENPL, BLM, KIF18A, CENPE, MCM4, BUB3, SUV39H2, MCM2, CDK2, PIF1, DNA2, region CENPO, CENPF, CHEK2, DSN1, H2AFX, MCM7, SUV39H1, MTBP, CBX3, RECQL4, KNTC1, PPP1CC, CENPP, CENPQ, PTGES3, NCAPD2, DYNLL1, SKA2, HAT1, NUP107, MCM5, MCM3, MSH2, BRCA2, NUP85, SSB, ITGB3BP, DMC1, INCENP, THOC3, XPO1, APEX1, XRCC5, KIF22, DCLRE1A, SEH1L, XRCC3, NSMCE2, RAD21. -

Loss of CIC Promotes Mitotic Dysregulation and Chromosome Segregation Defects
bioRxiv preprint doi: https://doi.org/10.1101/533323; this version posted January 29, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Loss of CIC promotes mitotic dysregulation and chromosome segregation defects. Suganthi Chittaranjan1, Jungeun Song1, Susanna Y. Chan1, Stephen Dongsoo Lee1, Shiekh Tanveer Ahmad2,3,4, William Brothers1, Richard D. Corbett1, Alessia Gagliardi1, Amy Lum5, Annie Moradian6, Stephen Pleasance1, Robin Coope1, J Gregory Cairncross2,7, Stephen Yip5,8, Emma Laks5,8, Samuel A.J.R. Aparicio5,8, Jennifer A. Chan2,3,4, Christopher S. Hughes1, Gregg B. Morin1,9, Veronique G. LeBlanc1, Marco A. Marra1,9* Affiliations 1 Canada's Michael Smith Genome Sciences Centre, BC Cancer, Vancouver, Canada 2 Arnie Charbonneau Cancer Institute, University of Calgary, Calgary, Canada 3 Department of Pathology & Laboratory Medicine, University of Calgary, Calgary, Canada 4 Alberta Children’s Hospital Research Institute, University of Calgary, Calgary, Canada 5 Department of Molecular Oncology, BC Cancer, Vancouver, Canada 6 California Institute of Technology, Pasadena, USA 7 Department of Clinical Neurosciences, University of Calgary, Calgary, Canada 8 Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada 9 Department of Medical Genetics, University of British Columbia, Vancouver, Canada Corresponding author Marco A. Marra Genome Sciences Centre BC Cancer 675 West 10th Avenue, Vancouver, BC Canada V5Z 1L3 E-mail: [email protected] 604-675-8162 1 bioRxiv preprint doi: https://doi.org/10.1101/533323; this version posted January 29, 2019. -

The Role of Epigenomics in Osteoporosis and Osteoporotic Vertebral Fracture
International Journal of Molecular Sciences Review The Role of Epigenomics in Osteoporosis and Osteoporotic Vertebral Fracture Kyoung-Tae Kim 1,2 , Young-Seok Lee 1,3 and Inbo Han 4,* 1 Department of Neurosurgery, School of Medicine, Kyungpook National University, Daegu 41944, Korea; [email protected] (K.-T.K.); [email protected] (Y.-S.L.) 2 Department of Neurosurgery, Kyungpook National University Hospital, Daegu 41944, Korea 3 Department of Neurosurgery, Kyungpook National University Chilgok Hospital, Daegu 41944, Korea 4 Department of Neurosurgery, CHA University School of medicine, CHA Bundang Medical Center, Seongnam-si, Gyeonggi-do 13496, Korea * Correspondence: [email protected]; Tel.: +82-31-780-1924; Fax: +82-31-780-5269 Received: 6 November 2020; Accepted: 8 December 2020; Published: 11 December 2020 Abstract: Osteoporosis is a complex multifactorial condition of the musculoskeletal system. Osteoporosis and osteoporotic vertebral fracture (OVF) are associated with high medical costs and can lead to poor quality of life. Genetic factors are important in determining bone mass and structure, as well as any predisposition for bone degradation and OVF. However, genetic factors are not enough to explain osteoporosis development and OVF occurrence. Epigenetics describes a mechanism for controlling gene expression and cellular processes without altering DNA sequences. The main mechanisms in epigenetics are DNA methylation, histone modifications, and non-coding RNAs (ncRNAs). Recently, alterations in epigenetic mechanisms and their activity have been associated with osteoporosis and OVF. Here, we review emerging evidence that epigenetics contributes to the machinery that can alter DNA structure, gene expression, and cellular differentiation during physiological and pathological bone remodeling. -

Intrinsic Disorder of the BAF Complex: Roles in Chromatin Remodeling and Disease Development
International Journal of Molecular Sciences Article Intrinsic Disorder of the BAF Complex: Roles in Chromatin Remodeling and Disease Development Nashwa El Hadidy 1 and Vladimir N. Uversky 1,2,* 1 Department of Molecular Medicine, Morsani College of Medicine, University of South Florida, 12901 Bruce B. Downs Blvd. MDC07, Tampa, FL 33612, USA; [email protected] 2 Laboratory of New Methods in Biology, Institute for Biological Instrumentation of the Russian Academy of Sciences, Federal Research Center “Pushchino Scientific Center for Biological Research of the Russian Academy of Sciences”, Pushchino, 142290 Moscow Region, Russia * Correspondence: [email protected]; Tel.: +1-813-974-5816; Fax: +1-813-974-7357 Received: 20 September 2019; Accepted: 21 October 2019; Published: 23 October 2019 Abstract: The two-meter-long DNA is compressed into chromatin in the nucleus of every cell, which serves as a significant barrier to transcription. Therefore, for processes such as replication and transcription to occur, the highly compacted chromatin must be relaxed, and the processes required for chromatin reorganization for the aim of replication or transcription are controlled by ATP-dependent nucleosome remodelers. One of the most highly studied remodelers of this kind is the BRG1- or BRM-associated factor complex (BAF complex, also known as SWItch/sucrose non-fermentable (SWI/SNF) complex), which is crucial for the regulation of gene expression and differentiation in eukaryotes. Chromatin remodeling complex BAF is characterized by a highly polymorphic structure, containing from four to 17 subunits encoded by 29 genes. The aim of this paper is to provide an overview of the role of BAF complex in chromatin remodeling and also to use literature mining and a set of computational and bioinformatics tools to analyze structural properties, intrinsic disorder predisposition, and functionalities of its subunits, along with the description of the relations of different BAF complex subunits to the pathogenesis of various human diseases. -

Tumorigenic Proteins Upregulated in the MYCN-Amplified IMR-32 Human Neuroblastoma Cells Promote Proliferation and Migration
INTERNATIONAL JOURNAL OF ONCOLOGY 52: 787-803, 2018 Tumorigenic proteins upregulated in the MYCN-amplified IMR-32 human neuroblastoma cells promote proliferation and migration HAYAT ZAATITI1*, JAD ABDALLAH2*, ZEINA NASR1, GEORGE KHAZEN3, ANTHONY SANDLER4 and TAMARA J. ABOU-ANTOUN2 1Department of Biology, Faculty of Sciences, University of Balamand, El-Koura; 2Department of Pharmaceutical Sciences, School of Pharmacy, and 3School of Arts and Sciences, Lebanese American University, Byblos 1102-2801, Lebanon; 4Sheikh Zayed Institute for Pediatric Surgical Innovation, Joseph E. Robert Jr. Center for Surgical Care, Children's National Medical Center, Washington, DC 20010, USA Received September 20, 2017; Accepted December 5, 2017 DOI: 10.3892/ijo.2018.4236 Abstract. Childhood neuroblastoma is one of the most and non-MYCN-amplified SK-N-SH human neuroblastoma common types of extra-cranial cancer affecting children with cells. Tumorigenic proteins, including fatty-acid binding a clinical spectrum ranging from spontaneous regression to protein 5 (FABP5), L1-cell adhesion molecule (L1-CAM), bacu- malignant and fatal progression. In order to improve the clin- loviral IAP repeat containing 5 [BIRC5 (survivin)] and high ical outcomes of children with high-risk neuroblastoma, it is mobility group protein A1 (HMGA1) were found to be signifi- crucial to understand the tumorigenic mechanisms that govern cantly upregulated in the IMR-32 compared to the SK-N-SH its malignant behaviors. MYCN proto-oncogene, bHLH tran- cells and mapped to highly tumorigenic pathways including, scription factor (MYCN) amplification has been implicated in MYC, MYCN, microtubule associated protein Tau (MAPT), the malignant, treatment-evasive nature of aggressive, high- E2F transcription factor 1 (E2F1), sterol regulatory element risk neuroblastoma. -

Itraq‑Based Proteomic Analysis of Endotoxin Tolerance Induced by Lipopolysaccharide
584 MOLECULAR MEDICINE REPORTS 20: 584-592, 2019 iTRAQ‑based proteomic analysis of endotoxin tolerance induced by lipopolysaccharide QIAN ZHANG1, YINGCHUN HU2, JING ZHANG1 and CUNLIANG DENG1 Departments of 1Infectious Diseases and 2Emergency, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan 646000, P.R. China Received August 25, 2018; Accepted February 15, 2019 DOI: 10.3892/mmr.2019.10264 Abstract. The purpose of the present study was to investigate Introduction the differentially expressed proteins between endotoxin toler- ance and sepsis. Cell models of an endotoxin tolerance group Sepsis is a serious healthcare concern due to its high costs (ET group) and sepsis group [lipopolysaccharide (LPS) group] and mortality rate (1). It is triggered mainly by Gram-negative were established using LPS and evaluated using ELISA and bacteria (2) and lipopolysaccharides (LPS) are the main flow cytometry methods. Differentially expressed proteins component of the outer membrane of Gram-negative bacteria. between the ET and the LPS groups were identified using Previous studies have demonstrated that LPS serve an important isobaric tags for relative and absolute quantitation (iTRAQ) role in sepsis, and can stimulate the mononuclear phagocytic analysis and evaluated by bioinformatics analysis. The expres- system to produce a large number of inflammatory factors sion of core proteins was detected by western blotting. It was and cause systemic inflammatory response, sepsis, infectious identified that the expression of tumor necrosis factor‑α and shock and multiple organ dysfunctions (3). It has been reported interleukin-6 was significantly decreased in the ET group that following low-dose LPS stimulation, the host can survive a compared with the LPS group. -

Metabolic Profiling of Cancer Cells Reveals Genome-Wide
ARTICLE https://doi.org/10.1038/s41467-019-09695-9 OPEN Metabolic profiling of cancer cells reveals genome- wide crosstalk between transcriptional regulators and metabolism Karin Ortmayr 1, Sébastien Dubuis1 & Mattia Zampieri 1 Transcriptional reprogramming of cellular metabolism is a hallmark of cancer. However, systematic approaches to study the role of transcriptional regulators (TRs) in mediating 1234567890():,; cancer metabolic rewiring are missing. Here, we chart a genome-scale map of TR-metabolite associations in human cells using a combined computational-experimental framework for large-scale metabolic profiling of adherent cell lines. By integrating intracellular metabolic profiles of 54 cancer cell lines with transcriptomic and proteomic data, we unraveled a large space of associations between TRs and metabolic pathways. We found a global regulatory signature coordinating glucose- and one-carbon metabolism, suggesting that regulation of carbon metabolism in cancer may be more diverse and flexible than previously appreciated. Here, we demonstrate how this TR-metabolite map can serve as a resource to predict TRs potentially responsible for metabolic transformation in patient-derived tumor samples, opening new opportunities in understanding disease etiology, selecting therapeutic treat- ments and in designing modulators of cancer-related TRs. 1 Institute of Molecular Systems Biology, ETH Zurich, Otto-Stern-Weg 3, CH-8093 Zurich, Switzerland. Correspondence and requests for materials should be addressed to M.Z. (email: [email protected]) NATURE COMMUNICATIONS | (2019) 10:1841 | https://doi.org/10.1038/s41467-019-09695-9 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-019-09695-9 ranscriptional regulators (TRs) are at the interface between implemented by the additional quantification of total protein the cell’s ability to sense and respond to external stimuli or abundance and is based on the assumption that protein content T 1 changes in internal cell-state .