The HMGA Proteins: a Myriad of Functions (Review)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Genetic Screening Identifies a Component of the SWI/SNF Complex, Arid1b As a Senescence Regulator
A genetic screening identifies a component of the SWI/SNF complex, Arid1b as a senescence regulator Sadaf Khan A thesis submitted to Imperial College London for the degree of Doctor in Philosophy MRC Clinical Sciences Centre Imperial College London, School of Medicine July 2013 Statement of originality All experiments included in this thesis were performed by myself unless otherwise stated. Copyright Declaration The copyright of this thesis rests with the author and is made available under a Creative Commons Attribution Non-Commercial No Derivatives license. Researchers are free to copy, distribute or transmit the thesis on the condition that they attribute it, that they do not use it for commercial purposes and that they do not alter, transform or build upon it. For any reuse or redistribution, researchers must make clear to others the license terms of this work. 2 Abstract Senescence is an important tumour suppressor mechanism, which prevents the proliferation of stressed or damaged cells. The use of RNA interference to identify genes with a role in senescence is an important tool in the discovery of novel cancer genes. In this work, a protocol was established for conducting bypass of senescence screenings, using shRNA libraries together with next-generation sequencing. Using this approach, the SWI/SNF subunit Arid1b was identified as a regulator of cellular lifespan in MEFs. SWI/SNF is a large multi-subunit complex that remodels chromatin. Mutations in SWI/SNF proteins are frequently associated with cancer, suggesting that SWI/SNF components are tumour suppressors. Here the role of ARID1B during senescence was investigated. Depletion of ARID1B extends the proliferative capacity of primary mouse and human fibroblasts. -

Identification of the Genes Up- and Down-Regulated by the High Mobility Group A1 (HMGA1) Proteins: Tissue Specificity of the HMGA1-Dependent Gene Regulation
[CANCER RESEARCH 64, 5728–5735, August 15, 2004] Identification of the Genes Up- and Down-Regulated by the High Mobility Group A1 (HMGA1) Proteins: Tissue Specificity of the HMGA1-Dependent Gene Regulation Josefina Martinez Hoyos,1 Monica Fedele,1 Sabrina Battista,1 Francesca Pentimalli,1,2 Mogens Kruhoffer,3 Claudio Arra,4 Torben F. Orntoft,3 Carlo Maria Croce,2 and Alfredo Fusco1 1Dipartimento di Biologia e Patologia Cellulare e Molecolare e/o Istituto di Endocrinologia ed Oncologia Sperimentale del CNR, Facolta` di Medicina e Chirurgia di Napoli, Universita` degli Studi di Napoli “Federico II,” Naples, Italy; 2Kimmel Cancer Center, Jefferson Medical College, Philadelphia, Pennsylvania; 3Department of Clinical Biochemistry, Aarhus University Hospital, Aarhus, Denmark; and 4Istituto Dei Tumori Di Napoli “Fondazione Pascale,” Naples, Italy. ABSTRACT To identify the differentiation pathways in which HMGA1 is in- volved and to assess the role of the HMGA1 proteins in development, High mobility group A (HMGA) proteins are chromatinic proteins that we generated embryonic stem (ES) cells in which one or both hmga1 do not have transcriptional activity per se, however, by interacting with alleles are disrupted. We reported recently that hmga1Ϫ/Ϫ ES cells the transcription machinery, they regulate, negatively or positively, the expression of several genes. We searched for genes regulated by HMGA1 generate less T-cell precursors than do wild-type ES cells after in proteins using microarray analysis in embryonic stem (ES) cells bearing vitro-specific differentiation. Indeed, they preferentially differentiate one or two disrupted hmga1 alleles. We identified 87 transcripts increased to B cells, probably consequent to decreased IL-2 expression and and 163 transcripts decreased of at least 4-fold in hmga1؊/؊ ES cells. -

Functional Compensation Among HMGN Variants Modulates the Dnase I Hypersensitive Sites at Enhancers
Downloaded from genome.cshlp.org on October 9, 2021 - Published by Cold Spring Harbor Laboratory Press Research Functional compensation among HMGN variants modulates the DNase I hypersensitive sites at enhancers Tao Deng,1,12 Z. Iris Zhu,2,12 Shaofei Zhang,1 Yuri Postnikov,1 Di Huang,2 Marion Horsch,3 Takashi Furusawa,1 Johannes Beckers,3,4,5 Jan Rozman,3,5 Martin Klingenspor,6,7 Oana Amarie,3,8 Jochen Graw,3,8 Birgit Rathkolb,3,5,9 Eckhard Wolf,9 Thure Adler,3 Dirk H. Busch,10 Valérie Gailus-Durner,3 Helmut Fuchs,3 Martin Hrabeˇ de Angelis,3,4,5 Arjan van der Velde,2,13 Lino Tessarollo,11 Ivan Ovcherenko,2 David Landsman,2 and Michael Bustin1 1Protein Section, Laboratory of Metabolism, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892, USA; 2Computational Biology Branch, National Center for Biotechnology Information, National Library of Medicine, Bethesda, Maryland 20892, USA; 3German Mouse Clinic, Institute of Experimental Genetics, Helmholtz Zentrum München, German Research Center for Environmental Health, 85764 Neuherberg, Germany; 4Experimental Genetics, Center of Life and Food Sciences Weihenstephan, Technische Universität München, 85354 Freising-Weihenstephan, Germany; 5German Center for Diabetes Research (DZD), 85764 Neuherberg, Germany; 6Molecular Nutritional Medicine, Technische Universität München, 85350 Freising, Germany; 7Center for Nutrition and Food Sciences, Technische Universität München, 85350 Freising, Germany; 8Institute of Developmental Genetics (IDG), 85764 -

Human Stem Cells from Single Blastomeres Reveal Pathways of Embryonic Or Trophoblast Fate Specification Tamara Zdravkovic1,2,3,4,5,‡, Kristopher L
© 2015. Published by The Company of Biologists Ltd | Development (2015) 142, 4010-4025 doi:10.1242/dev.122846 STEM CELLS AND REGENERATION RESEARCH ARTICLE Human stem cells from single blastomeres reveal pathways of embryonic or trophoblast fate specification Tamara Zdravkovic1,2,3,4,5,‡, Kristopher L. Nazor6,‡, Nicholas Larocque1,2,3,4,5, Matthew Gormley1,2,3,4,5, Matthew Donne1,2,3,7, Nathan Hunkapillar1,2,3,4,5, Gnanaratnam Giritharan8, Harold S. Bernstein4,9, Grace Wei4,10, Matthias Hebrok10, Xianmin Zeng11, Olga Genbacev1,2,3,4,5, Aras Mattis4,12, Michael T. McMaster4,5,13, Ana Krtolica8,*, Diana Valbuena14, Carlos Simón14, Louise C. Laurent6,15, Jeanne F. Loring6 and Susan J. Fisher1,2,3,4,5,7,§ ABSTRACT INTRODUCTION For many reasons, relatively little is known about human Mechanisms of initial cell fate decisions differ among species. To gain preimplantation development. The small number of cells makes insights into lineage allocation in humans, we derived ten human embryos of any species difficult to study. In humans, the technical embryonic stem cell lines (designated UCSFB1-10) from single difficulties are compounded by other challenges. Genetic variation blastomeres of four 8-cell embryos and one 12-cell embryo from a among individuals could contribute to developmental differences, a single couple. Compared with numerous conventional lines from well-appreciated phenomenon in the mouse (Dackor et al., 2009), blastocysts, they had unique gene expression and DNA methylation which is difficult to assess in humans owing to the limited patterns that were, in part, indicative of trophoblast competence. At a availability of embryos that are donated for research. -

High-Mobility Group A1 Proteins May Be Involved in Estrogen Receptor Status of Breast Cancer
3786 Editorial Commentary High-mobility group A1 proteins may be involved in estrogen receptor status of breast cancer Yoshihiro Harada, Kenji Ohe Department of Pharmacotherapeutics, Faculty of Pharmaceutical Sciences, Fukuoka University, Jonan-ku, Fukuoka, Japan Correspondence to: Kenji Ohe. Department of Pharmacotherapeutics, Faculty of Pharmaceutical Sciences, Fukuoka University, Building 17, 8-19-1 Nanakuma, Jonan-ku, Fukuoka 814-180, Japan. Email: [email protected]. Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research. The article did not undergo external peer review. Comment on: Gorbounov M, Carleton NM, Asch-Kendrick RJ, et al. High mobility group A1 (HMGA1) protein and gene expression correlate with ER-negativity and poor outcomes in breast cancer. Breast Cancer Res Treat 2020;179:25-35. Submitted Apr 27, 2020. Accepted for publication May 13, 2020. doi: 10.21037/tcr-20-1921 View this article at: http://dx.doi.org/10.21037/tcr-20-1921 The three types of breast cancer HMGA1 proteins: old and new proteins in breast cancer Breast cancer is one of the most common of cancers in woman. About 316,700 new cases were diagnosed as breast HMGA1 (previously called HMGI/Y) is a member of the cancer in US woman in 2019 and 41,760 were predicted to high-mobility group (HMG) proteins that were found from die from it (1). Breast cancer has a characteristic of therapy- their high mobility characteristics during polyacrylamide targeting receptors: hormone receptors (HR) that are electrophoresis of non-histone chromatin-associated estrogen receptor [ER: human ERα protein (NCBI protein proteins (3). -

Of Keeping and Tipping the Balance: Host Regulation and Viral Modulation of IRF3-Dependent IFNB1 Expression
viruses Review Of Keeping and Tipping the Balance: Host Regulation and Viral Modulation of IRF3-Dependent IFNB1 Expression Hella Schwanke 1,2 , Markus Stempel 1,2 and Melanie M. Brinkmann 1,2,* 1 Institute of Genetics, Technische Universität Braunschweig, 38106 Braunschweig, Germany; [email protected] (H.S.); [email protected] (M.S.) 2 Viral Immune Modulation Research Group, Helmholtz Centre for Infection Research, 38124 Braunschweig, Germany * Correspondence: [email protected]; Tel.: +49-531-6181-3069 Received: 15 June 2020; Accepted: 3 July 2020; Published: 7 July 2020 Abstract: The type I interferon (IFN) response is a principal component of our immune system that allows to counter a viral attack immediately upon viral entry into host cells. Upon engagement of aberrantly localised nucleic acids, germline-encoded pattern recognition receptors convey their find via a signalling cascade to prompt kinase-mediated activation of a specific set of five transcription factors. Within the nucleus, the coordinated interaction of these dimeric transcription factors with coactivators and the basal RNA transcription machinery is required to access the gene encoding the type I IFN IFNβ (IFNB1). Virus-induced release of IFNβ then induces the antiviral state of the system and mediates further mechanisms for defence. Due to its key role during the induction of the initial IFN response, the activity of the transcription factor interferon regulatory factor 3 (IRF3) is tightly regulated by the host and fiercely targeted by viral proteins at all conceivable levels. In this review, we will revisit the steps enabling the trans-activating potential of IRF3 after its activation and the subsequent assembly of the multi-protein complex at the IFNβ enhancer that controls gene expression. -

A Preliminary Transcriptome Analysis Suggests a Transitory Effect of Vitamin D on Mitochondrial Function in Obese Young Finnish Subjects
ID: 18-0537 8 5 E Einarsdottir et al. Effect of vitamin D on gene 8:5 559–570 expression RESEARCH A preliminary transcriptome analysis suggests a transitory effect of vitamin D on mitochondrial function in obese young Finnish subjects Elisabet Einarsdottir1,2,3,†, Minna Pekkinen1,4, Kaarel Krjutškov2,5, Shintaro Katayama3, Juha Kere1,2,3,6, Outi Mäkitie1,4,7,8 and Heli Viljakainen1,9 1Folkhälsan Institute of Genetics, University of Helsinki, Helsinki, Finland 2Molecular Neurology Research Program, University of Helsinki, Helsinki, Finland 3Department of Biosciences and Nutrition, Karolinska Institutet, Huddinge, Sweden 4Children’s Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland 5Competence Centre on Health Technologies, Tartu, Estonia 6School of Basic and Medical Biosciences, King’s College London, Guy’s Hospital, London, United Kingdom 7Department of Molecular Medicine and Surgery and Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden 8Department of Clinical Genetics, Karolinska University Hospital, Stockholm, Sweden 9Department of Food and Environmental Sciences, University of Helsinki, Helsinki, Finland Correspondence should be addressed to H Viljakainen: [email protected] †(E Einarsdottir is now at Department of Gene Technology, Science for Life Laboratory, KTH-Royal Institute of Technology, Solna, Sweden) Abstract Objective: The effect of vitamin D at the transcriptome level is poorly understood, Key Words and furthermore, it is unclear if it differs between obese and normal-weight subjects. f vitamin D The objective of the study was to explore the transcriptome effects of vitamin D f gene expression supplementation. f obesity Design and methods: We analysed peripheral blood gene expression using GlobinLock f transcriptome oligonucleotides followed by RNA sequencing in individuals participating in a 12-week f mitochondrial function randomised double-blinded placebo-controlled vitamin D intervention study. -
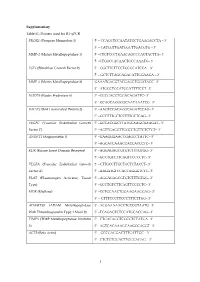
1 Supplementary Table S1. Primers Used for RT-Qpcr PROX1
Supplementary Table S1. Primers used for RT-qPCR PROX1 (Prospero Homeobox 1) 5’ – CCAGCTCCAATATGCTGAAGACCTA – 3’ 5’ – CATCGTTGATGGCTTGACGTG – 3‘ MMP-1 (Matrix Metallopeptidase 1) 5' –CTGTCCCTGAACAGCCCAGTACTTA– 3' 5' –CTGGCCACAACTGCCAAATG– 3' FGF2 (Fibroblast Growth Factor 2) 5′ - GGCTTCTTCCTGCGCATCCA – 3′ 5′ – GCTCTTAGCAGACATTGGAAGA – 3′ MMP-3 (Matrix Metallopeptidase 3) GAAATGAGGTACGAGCTGGATACC– 3’ 5’ –ATGGCTGCATCGATTTTCCT– 3’ NUDT6 (Nudix Hydrolase 6) 5’ –GGCGAGCTGGACAGATTC– 3’ 5’ –GCAGCAGGGGCAATAAATCG– 3’ BAIAP2 (BAI1 Associated Protein 2) 5’ –AAGTCCACAGGCAGATCCAG– 3’ 5’ –GCCTTTGCTCCTTTGCTCAG– 3’ VEGFC (Vascular Endothelial Growth 5’ –GCCACGGCTTATGCAAGCAAAGAT– 3’ Factor C) 5’ –AGTTGAGGTTGGCCTGTTCTCTGT– 3’ ANGPT1 (Angiopoietin 1) 5’ –GAAGGGAACCGAGCCTATTC– 3’ 5’ –AGCATCAAACCACCATCCTC– 3’ KDR (Kinase Insert Domain Receptor) 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ VEGFA (Vascular Endothelial Growth 5’ –CTTGCCTTGCTGCTCTACCT– 3’ Factor A) 5’ –AAGATGTCCACCAGGGTCTC– 3’ PLAT (Plasminogen Activator, Tissue 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ Type) 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ MDK (Midkine) 5’ –CCTGCAACTGGAAGAAGGAG– 3’ 5’ -- CTTTCCCTTCCCTTTCTTGG– 3’ ADAMTS9 (ADAM Metallopeptidase 5’ –ACGAAAAACCTGCCGTAATG– 3’ With Thrombospondin Type 1 Motif 9) 5’ –TCAGAGTCTCCATGCACCAG– 3’ TIMP3 (TIMP Metallopeptidase Inhibitor 5’ –CTGACAGGTCGCGTCTATGA– 3’ 3) 5’ –AGTCACAAAGCAAGGCAGGT– 3’ ACTB (Beta Actin) 5’ – GCCGAGGACTTTGATTGC – 3’ 5’– CTGTGTGGACTTGGGAGAG – 3’ 1 Figure S1. Efficient silencing of PROX1 in CGTH-W-1 and FTC-133 cells. Western blotting analysis shows a decrease in PROX1 protein level by targeting with siRNAs purchased from Santa Cruz (SC) and Sigma-Aldrich (SA) in both CGTH-W-1 and FTC-133 cell line. Beta-actin was used as a loading control of protein lysates. Figure S2. The tube formation assay. The silencing of PROX1 in CGTH-W-1 and FTC-133 cells enhances the angiogenesis in vitro of endothelial cells. HUVECs were cultured in 96-well plates coated with a semi-solid Matrigel. -

An Ontogenetic Switch Drives the Positive and Negative Selection of B Cells
An ontogenetic switch drives the positive and negative selection of B cells Xijin Xua, Mukta Deobagkar-Lelea, Katherine R. Bulla, Tanya L. Crockforda, Adam J. Meadb, Adam P. Cribbsc, David Simsc, Consuelo Anzilottia, and Richard J. Cornalla,1 aMedical Research Council Human Immunology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, OX3 9DS Oxford, United Kingdom; bMedical Research Council Molecular Haematology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, OX3 9DS Oxford, United Kingdom; and cMedical Research Council, Weatherall Institute of Molecular Medicine, Centre for Computational Biology, Weatherall Institute of Molecular Medicine, University of Oxford, OX3 9DS Oxford, United Kingdom Edited by Michael Reth, University of Freiburg, Freiburg, Germany, and approved January 6, 2020 (received for review September 3, 2019) + Developing B cells can be positively or negatively selected by self- BM HSCs increased CD5 B-1a B cell development (15), while antigens, but the mechanisms that determine these outcomes are expression of let-7b in FL pro-B cells blocked the development of incompletely understood. Here, we show that a B cell intrinsic B-1 B cells (17). These findings support the notion of hard-wired switch between positive and negative selection during ontogeny differences during ontogeny, but possibly downstream of the HSC is determined by a change from Lin28b to let-7 gene expression. commitment stage. Ectopic expression of a Lin28b transgene in murine B cells restored Several lines of evidence also suggest that B-1 B cells can un- the positive selection of autoreactive B-1 B cells by self-antigen in dergo positive selection, which is linked to their B cell receptor adult bone marrow. -

HMGB1 in Health and Disease R
Donald and Barbara Zucker School of Medicine Journal Articles Academic Works 2014 HMGB1 in health and disease R. Kang R. C. Chen Q. H. Zhang W. Hou S. Wu See next page for additional authors Follow this and additional works at: https://academicworks.medicine.hofstra.edu/articles Part of the Emergency Medicine Commons Recommended Citation Kang R, Chen R, Zhang Q, Hou W, Wu S, Fan X, Yan Z, Sun X, Wang H, Tang D, . HMGB1 in health and disease. 2014 Jan 01; 40():Article 533 [ p.]. Available from: https://academicworks.medicine.hofstra.edu/articles/533. Free full text article. This Article is brought to you for free and open access by Donald and Barbara Zucker School of Medicine Academic Works. It has been accepted for inclusion in Journal Articles by an authorized administrator of Donald and Barbara Zucker School of Medicine Academic Works. Authors R. Kang, R. C. Chen, Q. H. Zhang, W. Hou, S. Wu, X. G. Fan, Z. W. Yan, X. F. Sun, H. C. Wang, D. L. Tang, and +8 additional authors This article is available at Donald and Barbara Zucker School of Medicine Academic Works: https://academicworks.medicine.hofstra.edu/articles/533 NIH Public Access Author Manuscript Mol Aspects Med. Author manuscript; available in PMC 2015 December 01. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Mol Aspects Med. 2014 December ; 0: 1–116. doi:10.1016/j.mam.2014.05.001. HMGB1 in Health and Disease Rui Kang1,*, Ruochan Chen1, Qiuhong Zhang1, Wen Hou1, Sha Wu1, Lizhi Cao2, Jin Huang3, Yan Yu2, Xue-gong Fan4, Zhengwen Yan1,5, Xiaofang Sun6, Haichao Wang7, Qingde Wang1, Allan Tsung1, Timothy R. -

HMGA2 Promotes Adipogenesis by Activating C/EBP&Beta
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector Biochemical and Biophysical Research Communications 472 (2016) 617e623 Contents lists available at ScienceDirect Biochemical and Biophysical Research Communications journal homepage: www.elsevier.com/locate/ybbrc HMGA2 promotes adipogenesis by activating C/EBPb-mediated expression of PPARg * Yang Xi a, Wanjing Shen a, Lili Ma a, Ming Zhao a, Jiachen Zheng a, Shizhong Bu a, , ** Shinjiro Hino b, Mitsuyoshi Nakao b, c, a Diabetes Center, and Zhejiang Provincial Key Laboratory of Pathophysiology, Institute of Biochemistry and Molecular Biology, School of Medicine, Ningbo University, Ningbo 315211, China b Department of Medical Cell Biology, Institute of Molecular Embryology and Genetics, Kumamoto University, Kumamoto, 860-0811, Japan c Core Research for Evolutional Science and Technology (CREST), Japan Agency for Medical Research and Development, Tokyo, Japan article info abstract Article history: Adipogenesis is orchestrated by a highly ordered network of transcription factors including peroxisome- Received 1 March 2016 proliferator activated receptor-gamma (PPARg) and CCAAT-enhancer binding protein (C/EBP) family Accepted 6 March 2016 proteins. High mobility group protein AT-hook 2 (HMGA2), an architectural transcription factor, has been Available online 8 March 2016 reported to play an essential role in preadipocyte proliferation, and its overexpression has been impli- cated in obesity in mice and humans. However, the direct role of HMGA2 in regulating the gene Keywords: expression program during adipogenesis is not known. Here, we demonstrate that HMGA2 is required for Adipogenesis C/EBPb-mediated expression of PPARg, and thus promotes adipogenic differentiation. We observed a HMGA2 C/EBPb transient but marked increase of Hmga2 transcript at an early phase of differentiation of mouse 3T3-L1 PPARg preadipocytes. -
Supplemental Data.Pdf
Table S1. Summary of sequencing results A. DNase‐seq data % Align % Mismatch % >=Q30 bases Sample ID # Reads % unique reads (PF) Rate (PF) (PF) WT_1 84,301,522 86.76 0.52 93.34 96.30 WT_2 98,744,222 84.97 0.51 93.75 89.94 Hmgn1‐/‐_1 79,620,656 83.94 0.86 90.58 88.65 Hmgn1‐/‐_2 62,673,782 84.13 0.87 91.30 89.18 Hmgn2‐/‐_1 87,734,440 83.49 0.71 91.81 90.00 Hmgn2‐/‐_2 82,498,808 83.25 0.69 92.73 90.66 Hmgn1‐/‐n2‐/‐_1 71,739,638 68.51 2.31 81.11 89.22 Hmgn1‐/‐n2‐/‐_2 74,113,682 68.19 2.37 81.16 86.57 B. ChIP‐seq data Histone % Align % Mismatch % >=Q30 % unique Genotypes # Reads marks (PF) Rate (PF) bases (PF) reads H3K4me1 100670054 92.99 0.28 91.21 87.29 H3K4me3 67064272 91.97 0.35 89.11 27.15 WT H3K27ac 90,340,242 93.57 0.28 95.02 89.80 input 111,292,572 78.24 0.55 96.07 86.99 H3K4me1 84598176 92.34 0.33 91.2 81.69 H3K4me3 90032064 92.19 0.44 88.76 15.81 Hmgn1‐/‐n2‐/‐ H3K27ac 86,260,526 93.40 0.29 94.94 87.49 input 78,142,334 78.47 0.56 95.82 81.11 C. MNase‐seq data % Mismatch % >=Q30 bases % unique Sample ID # Reads % Align (PF) Rate (PF) (PF) reads WT_1_Extensive 45,232,694 55.23 1.49 90.22 81.73 WT_1_Limited 105,460,950 58.03 1.39 90.81 79.62 WT_2_Extensive 40,785,338 67.34 1.06 89.76 89.60 WT_2_Limited 105,738,078 68.34 1.05 90.29 85.96 Hmgn1‐/‐n2‐/‐_1_Extensive 117,927,050 55.74 1.49 89.50 78.01 Hmgn1‐/‐n2‐/‐_1_Limited 61,846,742 63.76 1.22 90.57 84.55 Hmgn1‐/‐n2‐/‐_2_Extensive 137,673,830 60.04 1.30 89.28 78.99 Hmgn1‐/‐n2‐/‐_2_Limited 45,696,614 62.70 1.21 90.71 85.52 D.