Seed Morphology and Testa Ultrastructure in Allium Stipitatum Complex (Amaryllidaceae; Allioideae) and Their Systematic Significance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

The Republic of Tajikistan Ministry of Energy and Industry
The Republic of Tajikistan Ministry of Energy and Industry DATA COLLECTION SURVEY ON THE INSTALLMENT OF SMALL HYDROPOWER STATIONS FOR THE COMMUNITIES OF KHATLON OBLAST IN THE REPUBLIC OF TAJIKISTAN FINAL REPORT September 2012 Japan International Cooperation Agency NEWJEC Inc. E C C CR (1) 12-005 Final Report Contents, List of Figures, Abbreviations Data Collection Survey on the Installment of Small Hydropower Stations for the Communities of Khatlon Oblast in the Republic of Tajikistan FINAL REPORT Table of Contents Summary Chapter 1 Preface 1.1 Objectives and Scope of the Study .................................................................................. 1 - 1 1.2 Arrangement of Small Hydropower Potential Sites ......................................................... 1 - 2 1.3 Flowchart of the Study Implementation ........................................................................... 1 - 7 Chapter 2 Overview of Energy Situation in Tajikistan 2.1 Economic Activities and Electricity ................................................................................ 2 - 1 2.1.1 Social and Economic situation in Tajikistan ....................................................... 2 - 1 2.1.2 Energy and Electricity ......................................................................................... 2 - 2 2.1.3 Current Situation and Planning for Power Development .................................... 2 - 9 2.2 Natural Condition ............................................................................................................ -

Complete Chloroplast Genomes Shed Light on Phylogenetic
www.nature.com/scientificreports OPEN Complete chloroplast genomes shed light on phylogenetic relationships, divergence time, and biogeography of Allioideae (Amaryllidaceae) Ju Namgung1,4, Hoang Dang Khoa Do1,2,4, Changkyun Kim1, Hyeok Jae Choi3 & Joo‑Hwan Kim1* Allioideae includes economically important bulb crops such as garlic, onion, leeks, and some ornamental plants in Amaryllidaceae. Here, we reported the complete chloroplast genome (cpDNA) sequences of 17 species of Allioideae, fve of Amaryllidoideae, and one of Agapanthoideae. These cpDNA sequences represent 80 protein‑coding, 30 tRNA, and four rRNA genes, and range from 151,808 to 159,998 bp in length. Loss and pseudogenization of multiple genes (i.e., rps2, infA, and rpl22) appear to have occurred multiple times during the evolution of Alloideae. Additionally, eight mutation hotspots, including rps15-ycf1, rps16-trnQ-UUG, petG-trnW-CCA , psbA upstream, rpl32- trnL-UAG , ycf1, rpl22, matK, and ndhF, were identifed in the studied Allium species. Additionally, we present the frst phylogenomic analysis among the four tribes of Allioideae based on 74 cpDNA coding regions of 21 species of Allioideae, fve species of Amaryllidoideae, one species of Agapanthoideae, and fve species representing selected members of Asparagales. Our molecular phylogenomic results strongly support the monophyly of Allioideae, which is sister to Amaryllioideae. Within Allioideae, Tulbaghieae was sister to Gilliesieae‑Leucocoryneae whereas Allieae was sister to the clade of Tulbaghieae‑ Gilliesieae‑Leucocoryneae. Molecular dating analyses revealed the crown age of Allioideae in the Eocene (40.1 mya) followed by diferentiation of Allieae in the early Miocene (21.3 mya). The split of Gilliesieae from Leucocoryneae was estimated at 16.5 mya. -

Analysis of Essential Oil from Leaves and Bulbs of Allium Atroviolaceum
Brief Communication and Method report 2020;3(1):e8 Analysis of essential oil from leaves and Bulbs of Allium atroviolaceum a a b c* Parniyan Sebtosheikh , Mahnaz Qomi , Shima Ghadami , Faraz Mojab a. Faculty of Pharmaceutical Chemistry, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran. b. Faculty of Pharmacy, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran. c. School of Pharmacy and Pharmaceutical Sciences Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Article Info: Abstract: Received: September 2020 Introduction: Medicinal plants used in traditional medicine as prevention and treatment Accepted: September 2020 of disease and illness or use in foods, has a long history. Plants belonging to genera Published online: Allium have widely been acquired as food and medicine. In many countries, including September 2020 Iran, a variety of species of the genus Allium such as garlic, onions, leeks, shallots, etc use for food and medicinal uses. Methods and Results: The leaves and bulbs of Allium atroviolaceum, collected from * Corresponding Author: Borujerd (Lorestan Province, Iran) in May 2015 and their essential oils of were obtained Faraz Mojab Email: [email protected] by hydro-distillation. The oils were analyzed by gas chromatography coupled with mass spectrometry (GC/MS) and their chemical composition was identified. The major constituents of A. atroviolaceum leaves oil were dimethyl trisulfide (59.0%), ethyl linolenate (12.4%), phytol (11.4%) and in bulb oil were methyl methyl thiomethyl disulfide (61.3%), dimethyl trisulfide (15.1%) and methyl allyl disulfide (4.3%). The major constituents of both essential oils are sulfur compounds. Conclusion: The results of the present study can help to increase of our information about composition of an edible herb in Iran. -

Nematicidal, Phytotoxic and Brine Shrimp Lethality Activity of Some Allium Species and Their Bioactive Sulfur Compounds
Nematicidal, Phytotoxic and Brine Shrimp Lethality Activity of Some Allium Species and Their Bioactive Sulfur Compounds Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften (Dr. rer. nat.) dem Fachbereich Pharmazie der Philipps-Universität Marburg vorgelegt von Sevda Jivishova aus Baku, Aserbaidschan Marburg/Lahn Jahr 2018 Erstgutachter: Prof. Dr. Michael Keusgen Zweitgutachter: Prof. Dr. Shuming Li Eingereicht am ........................ Tag der ndlichen Prüfung am 21.12.2016 Hochschulkennziffer: 1180 Dedicated to my husband and life partner Emil, our little hearts-children Said and Esma, my beloved parents and my proud brother Pervin, to the supporting parents-in-law and brother-in-law Orkhan. If I have seen further than others, it is by standing upon the shoulders of giants. Isaac Newton TABLE OF CONTENTS TABLE OF CONTENTS ........................................................................................... 1 Acknowledgments .................................................................................................... 5 List of Figures........................................................................................................... 7 List of Tables .......................................................................................................... 10 List of Abbreviations ............................................................................................... 11 Summary ................................................................................................................ 14 -

ISTA List of Stabilized Plant Names 7Th Edition
ISTA List of Stabilized Plant Names th 7 Edition ISTA Nomenclature Committee Chair: Dr. M. Schori Published by All rights reserved. No part of this publication may be The Internation Seed Testing Association (ISTA) reproduced, stored in any retrieval system or transmitted Zürichstr. 50, CH-8303 Bassersdorf, Switzerland in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior ©2020 International Seed Testing Association (ISTA) permission in writing from ISTA. ISBN 978-3-906549-77-4 ISTA List of Stabilized Plant Names 1st Edition 1966 ISTA Nomenclature Committee Chair: Prof P. A. Linehan 2nd Edition 1983 ISTA Nomenclature Committee Chair: Dr. H. Pirson 3rd Edition 1988 ISTA Nomenclature Committee Chair: Dr. W. A. Brandenburg 4th Edition 2001 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 5th Edition 2007 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 6th Edition 2013 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 7th Edition 2019 ISTA Nomenclature Committee Chair: Dr. M. Schori 2 7th Edition ISTA List of Stabilized Plant Names Content Preface .......................................................................................................................................................... 4 Acknowledgements ....................................................................................................................................... 6 Symbols and Abbreviations .......................................................................................................................... -

Effect of Extract of Allium Stipitatum on Excisional Wound Healing in Rats Amin Mohammadi-Rika1, Mandana Beigi-Boroujeni2, Asghar Rajabzadeh2, Leila Zarei2,3*
Iran J Vet Surg 2021; 16(1); Serial No: 34; Pages: 5-11 Iranian Veterinary Surgery Association Iranian Journal of Veterinary Surgery Journal homepage: www.ivsajournals.com Original Article Effect of Extract of Allium stipitatum on Excisional Wound Healing in Rats Amin Mohammadi-Rika1, Mandana Beigi-Boroujeni2, Asghar Rajabzadeh2, Leila Zarei2,3* 1 Student Research Committee, Lorestan University of Medical Sciences, Khorramabad, Iran. 2 Department of Anatomical Sciences, Faculty of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran. 3 Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran. ARTICLE INFO ABSTRACT The aim of the present study was to assess the wound-healing activity of extract of Allium Article History: stipitatum. Thirty-six male Wistar rats were used in this study. The rats weighing Received 16 June 2020 approximately 160-180 g and seven weeks of age were randomized into three groups of 12 Revised 20 September 2020 rats each: Control surgery group (Control) including the creation of wounds and no treatment, Accepted 28 September 2020 base formulation groups positive (POS) with the creation of wounds and application of base Online 28 September 2020 formulation ointment, treatment group 1 (T1) with 2 g of powder extract of the plant material in the ointment. A wound was induced by an excisional based wound model in male rats. The Keywords: mature green leaves of Allium stipitatum were collected and authenticated. Extractions of dried leaves were carried out. For wound-healing activity, the extracts were applied topically Herbal extract in the form of ointment and compared to control groups. The healing of the wound was Allium stipitatum assessed based on the wound area, histomorphometry, and hydroxyproline estimation Wound healing studies. -
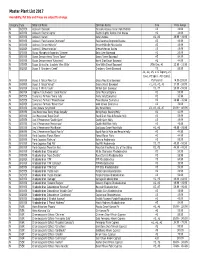
Master Plant List 2017.Xlsx
Master Plant List 2017 Availability, Pot Size and Prices are subject to change. Category Type Botanical Name Common Name Size Price Range N BREVER Azalea X 'Cascade' Cascade Azalea (Glenn Dale Hybrid) #3 49.99 N BREVER Azalea X 'Electric Lights' Electric Lights Double Pink Azalea #2 44.99 N BREVER Azalea X 'Karen' Karen Azalea #2, #3 39.99 - 49.99 N BREVER Azalea X 'Poukhanense Improved' Poukhanense Improved Azalea #3 49.99 N BREVER Azalea X 'Renee Michelle' Renee Michelle Pink Azalea #3 49.99 N BREVER Azalea X 'Stewartstonian' Stewartstonian Azalea #3 49.99 N BREVER Buxus Microphylla Japonica "Gregem' Baby Gem Boxwood #2 29.99 N BREVER Buxus Sempervirens 'Green Tower' Green Tower Boxwood #5 64.99 N BREVER Buxus Sempervirens 'Katerberg' North Star Dwarf Boxwood #2 44.99 N BREVER Buxus Sinica Var. Insularis 'Wee Willie' Wee Willie Dwarf Boxwood Little One, #1 13.99 - 21.99 N BREVER Buxus X 'Cranberry Creek' Cranberry Creek Boxwood #3 89.99 #1, #2, #5, #15 Topiary, #5 Cone, #5 Spiral, #10 Spiral, N BREVER Buxus X 'Green Mountain' Green Mountain Boxwood #5 Pyramid 14.99-299.99 N BREVER Buxus X 'Green Velvet' Green Velvet Boxwood #1, #2, #3, #5 17.99 - 59.99 N BREVER Buxus X 'Winter Gem' Winter Gem Boxwood #5, #7 59.99 - 99.99 N BREVER Daphne X Burkwoodii 'Carol Mackie' Carol Mackie Daphne #2 59.99 N BREVER Euonymus Fortunei 'Ivory Jade' Ivory Jade Euonymus #2 35.99 N BREVER Euonymus Fortunei 'Moonshadow' Moonshadow Euonymus #2 29.99 - 35.99 N BREVER Euonymus Fortunei 'Rosemrtwo' Gold Splash Euonymus #2 39.99 N BREVER Ilex Crenata 'Sky Pencil' -

Survey of Wild Food Plants for Human Consumption in Geçitli (Hakkari, Turkey)
Indian Journal of Traditional Knowledge Vol. 14(2), April 2015, pp. 183-190 Survey of wild food plants for human consumption in Geçitli (Hakkari, Turkey) İdris Kaval1, Lütfi Behçet2 & Uğur Çakilcioğlu3* 1Yuzuncu Yıl University, Department of Biology, Van 65000, Turkey; 2Bingöl University, Department of Biology, Bingöl 12000, Turkey; 3Tunceli University, Pertek Sakine Genç Vocational School, Pertek, Tunceli 62500, Turkey E-mails: [email protected]; [email protected]; [email protected] Received 15 July 2014, revised 22 January 2015 This study aims to record accumulation of knowledge on plants which are used as food by native people of Geçitli (Hakkari, Turkey) that has a rich culture and a very natural environment. In addition, the medical uses of these plants were compiled from the literature. Study area was located on the East of Anatolian diagonal, in the Eastern Anatolia region. Field study was carried out over a period of approximately two years (2008-2010). During this period, 84 vascular plant taxa were collected. The plants were pressed in the field and prepared for identification. A total of 84 food plants belonging to 30 families were identified in the region. In the study being conducted, use of wild plants as food points out interest of people in Geçitli in wild plants. The fact that a large proportion of edible plants are also being used for medicinal purposes indicates that the use of wild plants has a high potential in the region. The present study shows that further ethnobotanical investigations are worthy to be carried out in Turkey, where most of knowledge on popular food plants are still to discover. -

Alliums of All Shapes & Sizes’
Tips From Trecanna Trecanna Nursery is a family-run plant nursery owned by Mark & Karen Wash and set on the Cornish slopes of the Tamar Valley, specialising in unusual bulbs & perennials, Crocosmias and other South African plants. Each month Mark will write a feature on some of his very favourite plants. Trecanna Nursery is now open from Wednesday to Saturday throughout the year, from 10am to 5pm, (or phone to arrange a visit at other times). There is a wide range of unusual bulbs, herbaceous plants and hardy South African plants including the largest selection of Crocosmia in the South. We are located approx. 2 miles north of Gunnislake. Follow the brown tourist signs from the A390, Callington to Gunnislake road. Tel: 01822 834680. Email: [email protected] Talks to garden clubs and societies. ‘Alliums Of All Shapes & Sizes’ Last year I covered a number of fabulous Alliums that you plant and enjoy in your garden, however as there are so many excellent varieties to choose from, I have decided to look at some more - particularly as May & June is when the vast majority of them burst into flower. The main displays of spring flowering bulbs, including narcissi and most tulips, are just coming to an end in May. The Alliums fulfil a valuable task, bridging the gap between Spring & Summer before many of our herbaceous plants come into their prime. There are a vast array of wild Alliums in existence coming from areas such as Asia, North America and Europe – in fact the wild species number over 700 and with all the hybrids that have been bred over the years the choice is now literally thousands. -

Agricultural Management and Environmental Requirements for Production of True Shallot Seeds – a Review
Advances in Plants & Agriculture Research Review Article Open Access Agricultural management and environmental requirements for production of true shallot seeds – a review Abstract Volume 9 Issue 2 - 2019 Shallots widely grow in very cold to moderate cold temperate climates at high Omid Askari-Khorasgani,1 Mohammad elevations. Due to low seed production rate and the lack of seed producing cultivars, 2 in most cases, shallots are vegetatively propagated by using bulb material. Cultivation Pessarakli 1Young Researchers and Elite Club, Department of Horticulture, of high-quality true shallot seeds (TSS) under suitable environmental conditions and College of Agriculture and Natural Resources, Isfahan agricultural management have several advantages over bulb materials, such as, smaller (Khorasgan) Branch, Islamic Azad University, Iran quantity of planting materials, easier transportation, long-term storing capacity, 2Professor, School of Plant Sciences, College of Agriculture and production of large and disease-free bulbs, and greater yield. Most studies focus on Life Sciences, The University of Arizona, USA improving shallot bulb production and understanding the agricultural methods for improving yield and quality of TSS require more attention in the future. Hence, this Correspondence: Mohammad Pessarakli, Editor-in-Chief, review discusses the most efficient methods for production of TSS. Advances in Plants & Agriculture Research, Professor, School of Plant Sciences, College of Agriculture and Life Sciences, The Keywords: Allium, Mooseer, -

Towards a Molecular Understanding of the Biosynthesis of Amaryllidaceae Alkaloids in Support of Their Expanding Medical Use
Int. J. Mol. Sci. 2013, 14, 11713-11741; doi:10.3390/ijms140611713 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Towards a Molecular Understanding of the Biosynthesis of Amaryllidaceae Alkaloids in Support of Their Expanding Medical Use Adam M. Takos and Fred Rook * Plant Biochemistry Laboratory, Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg, Denmark; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +45-3533-3343; Fax: +45-3533-3300. Received: 28 April 2013; in revised form: 26 May 2013 / Accepted: 27 May 2013 / Published: 31 May 2013 Abstract: The alkaloids characteristically produced by the subfamily Amaryllidoideae of the Amaryllidaceae, bulbous plant species that include well know genera such as Narcissus (daffodils) and Galanthus (snowdrops), are a source of new pharmaceutical compounds. Presently, only the Amaryllidaceae alkaloid galanthamine, an acetylcholinesterase inhibitor used to treat symptoms of Alzheimer’s disease, is produced commercially as a drug from cultivated plants. However, several Amaryllidaceae alkaloids have shown great promise as anti-cancer drugs, but their further clinical development is restricted by their limited commercial availability. Amaryllidaceae species have a long history of cultivation and breeding as ornamental bulbs, and phytochemical research has focussed on the diversity in alkaloid content and composition. In contrast to the available pharmacological and phytochemical data, ecological, physiological and molecular aspects of the Amaryllidaceae and their alkaloids are much less explored and the identity of the alkaloid biosynthetic genes is presently unknown. An improved molecular understanding of Amaryllidaceae alkaloid biosynthesis would greatly benefit the rational design of breeding programs to produce cultivars optimised for the production of pharmaceutical compounds and enable biotechnology based approaches.