Esting Resemblances Between the Avian and the Reptilian and the Mam- Malian Nuclear Pattern in This Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

University International
INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help you understand markings or notations which may appear on this reproduction. 1. The sign or “target” for pages apparently lacking from the document photographed is “Missing Page(s)”. If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure you of complete continuity. 2. When an image on the film is obliterated with a round black mark it is an indication that the film inspector noticed either blurred copy because of movement during exposure, or duplicate copy. Unless we meant to delete copyrighted materials that should not have been filmed, you will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., is part of the material being photo graphed the photographer has followed a definite method in “sectioning” the material. It is customary to begin filming at the upper left hand corner of a large sheet and to continue from left to right in equal sections with small overlaps. If necescary, sectioning is continued again—beginning below the first row and continuing on until complete. 4. For any illustrations that cannot be reproduced satisfactorily by xerography, photographic prints can be purchased at additional cost and tipped into your xerographic copy. -

The Nuclear Pattern of the Non-Tectal Portions of the Midbrain and Isthmus in Primates
THE NUCLEAR PATTERN OF THE NON-TECTAL PORTIONS OF THE MIDBRAIN AND ISTHMUS IN PRIMATES ELIZABETH C. CROSBY AND RUSSELL T. WOODBURNE Department of Anatomy, University of Michigan FOURTEEN PLATES (TWENTY-THREE FIGURES) INTRODUCTION The various nuclear groups in the midbrain of primates show marked resemblances to their homologues in subprimate forms, although certain regions are characterized by a marked increase and others by a definite decrease in development. The literature dealing with special regions is considerable in amount and some of it is of relatively early date. That pertinent to the present consideration of these regions has been reviewed under the account of the general literature placed at the end of this series of papers. Attention is called here to a few contributions that have been particularly helpful in the preparation of the primate descriptions. Among such are the studies of Ziehen on various regions of the midbrain, particularly his account of the periventricular regions, and the descriptive analysis of Ingram and Ranson ('35) of the nucleus of Darkschewitsch and the interstitial nucleus of the medial longitudinal fasciculus, which confirmed Stengel's ( '24) earlier account. There are innumerable figures and descrip- tions of various parts of the oculomotor and trochlear gray in primates. Among these may be mentioned the work of Panegrossi ('04), Ram6n y Cajal ('ll), Brouwer ('18, and elsewhere), Le Gros Clark ( '26) and Mingazzini ( '28). The differences in nomenclature with respect to the periventricular gray of caudal midbrain and isthmus regions are indicated 441 442 G. CARL HUBER ET AL. to some extent on the figures of the human brain as well as considered in the general literature. -

Lecture (6) Internal Structures of the Brainstem.Pdf
Internal structures of the Brainstem Neuroanatomy block-Anatomy-Lecture 6 Editing file Objectives At the end of the lecture, students should be able to: ● Distinguish the internal structure of the components of the brain stem in different levels and the specific criteria of each level. 1. Medulla oblongata (closed, mid and open medulla) 2. Pons (caudal and rostral). 3. Midbrain ( superior and inferior colliculi). Color guide ● Only in boys slides in Green ● Only in girls slides in Purple ● important in Red ● Notes in Grey Medulla oblongata Caudal (Closed) Medulla Traversed by the central canal Motor decussation (decussation of the pyramids) ● Formed by pyramidal fibers, (75-90%) cross to the opposite side ● They descend in the lateral white column of the spinal cord as the lateral corticospinal tract. ● The uncrossed fibers form the ventral corticospinal tract Trigeminal sensory nucleus. ● it is the larger sensory nucleus. ● The Nucleus Extends Through the whole length of the brainstem and its note :All CN V afferent sensory information enters continuation of the substantia gelatinosa of the spinal cord. the brainstem through the nerve itself located in the pons. Thus, to reach the spinal nucleus (which ● It lies in all levels of M.O, medial to the spinal tract of the trigeminal. spans the entire brain stem length) in the Caudal ● It receives pain and temperature from face, forehead. Medulla those fibers have to "descend" in what's known as the Spinal Tract of the Trigeminal ● Its tract present in all levels of M.O. is formed of descending (how its sensory and descend?see the note) fibers that terminate in the trigeminal nucleus. -

Trochlear Nerve Nucleus in Albino Rat
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861. Volume 5, Issue 5 (Mar.- Apr. 2013), PP 65-68 www.iosrjournals.org Trochlear Nerve Nucleus in Albino rat Singh A., Khanna J. & Bharihoke V. Department of Anatomy, University College of Medical Sciences & Guru Teg Bahadur Hospital, Delhi India Abstract: A study of the trochlear nerve nucleus and its course within the brain is reported based on histological and fluorescent tract tracing techniques. Twenty inbred adult Wistar albino rats weighing between 150 to 250 gm of either sex were taken in the study. The localization of the nucleus giving rise to the nerve supplying the superior oblique muscle was done by using fluorescent dyes, Fast blue and Diamidino yellow. Fast blue was applied to the trochlear nerve of the right side and the Diamidino yellow was applied to the nerve of the left side in each animal. Animals were sacrificed after appropriate survival period. The labeled neurons were localized in the fourth nerve nucleus in the midbrain with the help of a Ziess fluorescence microscope .The majority of the fibers of the trochlear nerve cross to the opposite side and a few fibers remain ipsilateral. Many neurons were labeled with both the dyes indicating bilateral innervation. The nucleus was studied in paraffin sections stained with Kluver Barrera and Marshland, Glees and Erikson’s stain to trace the nerve fibers within the midbrain. Key words: Fluorescent tract tracing techniques, midbrain, superior medullary vellum, trochlear nerve nucleus, Diamidino yellow, Fast blue I. Introduction The basis of origin of the trochlear nerve from the posterior aspect of the midbrain and its crossing before exiting from the brain remains an enigma. -

Cranial Nerves and Their Nuclei
CranialCranial nervesnerves andand theirtheir nucleinuclei 鄭海倫鄭海倫 整理整理 Cranial Nerves Figure 13.4a Location of the cranial nerves • Anterior cranial fossa: C.N. 1–2 • Middle cranial fossa: C.N. 3-6 • Posterior cranial fossa: C.N. 7-12 FunctionalFunctional componentscomponents inin nervesnerves • General Somatic Efferent • Special Visceral Afferent •GSE GSA GVE GVA • (SSE) SSA SVE SVA Neuron columns in the embryonic spinal cord * The floor of the 4th ventricle in the embryonic rhombencephalon Sp: special sensory B:branchial motor Ss: somatic sensory Sm: somataic motor Vi: visceral sensory A: preganglionic autonomic (visceral motor) • STT: spinothalamic tract • CST: corticospinal tract • ML: medial lemniscus Sensory nerve • Olfactory (1) •Optic (2) • Vestibulocochlear (8) Motor nerve • Oculomotor (3) • Trochlear (4) • Abducens (6) • Accessory (11) • Hypoglossal (12) Mixed nerve • Trigeminal (5) • Facial (7) • Glossopharyngeal (9) • Vagus (10) Innervation of branchial muscles • Trigemial • Facial • Glossopharyngeal • Vagus Cranial Nerve I: Olfactory Table 13.2(I) Cranial Nerve II: Optic • Arises from the retina of the eye • Optic nerves pass through the optic canals and converge at the optic chiasm • They continue to the thalamus (lateral geniculate body) where they synapse • From there, the optic radiation fibers run to the visual cortex (area 17) • Functions solely by carrying afferent impulses for vision Cranial Nerve II: Optic Table 13.2(II) Cranial Nerve III: Oculomotor • Fibers extend from the ventral midbrain, pass through the superior orbital fissure, and go to the extrinsic eye muscles • Functions in raising the eyelid, directing the eyeball, constricting the iris, and controlling lens shape Cranial Nerve III: Oculomotor Table 13.2(III) 1.Oculomotor nucleus (GSE) • Motor to ocular muscles: rectus (superior對側, inferior同側and medial同 側),inferior oblique同側, levator palpebrae superioris雙側 2. -

BRAIN, Hnd CEREBELLAR CENTERS and FIBER TRACTS IS BIRDS1
A CONSIDERATION OF CERTAIN BULBAB, R111)- BRAIN, hND CEREBELLAR CENTERS AND FIBER TRACTS IS BIRDS1 ESTHER ELICK SANDERS Lnboratory of Comparative Xeurology, Department of Anatomy, University of Michigan FIFTEEN FIGURES CONTENTS Introduction . , . 156 Materials and methods . 167 Literature .. ...,..... 157 Description of nuclear . 165 Nuclei aiid root fibers of the eye-muscle nerves . .. .. 163 Oculomotor nuclei aiid root fibers . 166 Trochlear nucleus and root fibers .. , . ,.. ..... .. 168 Abdueens nuclei arid root fibers , ............. 1g9 Fiber connections of the eye-muscle nuclei . , . 171 Tecto-bulbar tract . 173 Dorsal tecto-bulbar tract . , . , . 17.5 Ventral tecto-bulbar tract . ............... 176 Trigemiiial nuclei and root fibers . , . , . 177 Sensory nudei of the trigeminal 177 Motor nuclei of the trigcminal . 179 Cutaneous sensory root of the trigeminal . 183 Mesencephalic and motor roots of the trigeminal . I . 183 Secondary connections of the t.rigemiii 153 Internuclear fibers from sensory trigemiiial nuclci 183 Trigemino-cerebellar connections . , . 183 Secondary ascending trigemiual bundle, (trigemino-mesencephalic tract) ................................................ 186 Quinto-frontal tract . .......... 187 Cerebello-motorius fibers . , . , . , . 189 Nuclei and root fibers of the facial nerve . 189 Sensory nucleus of the facial nerve ........................... 189 Motor nuclei of the facial nerve , , . ._.. 189 Root fibers of the facial nerve . 190 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Unirersity of Michigan. 155 THE JOURNAL OF COYPARATIVE NEUROLOGY, VOL. 49, NO. 1 156 ESTHER BLICK SAWDERS Suclear centers and root fibers of the cochlear nerve ................ 190 Nuclear centers of the cochlear nerve ........ ............ 190 194 195 Vestibular nuclei aiid root fibers .... 197 Vestibular nuclei ............................................ 197 Vestibular root fibers ................... .......... 204 Secondary connections of the vestibular nerve .................... -

Ycine As an Inhibitory Neurotransmitter of Vestibular, Reticular, and Prepositus Hypoglossi Neurons That Project to the Cat Abducens Nucleus
The Journal of Neuroscience, August 1989, g(8): 2718-2736 Evidence for G!ycine as an Inhibitory Neurotransmitter of Vestibular, Reticular, and Prepositus Hypoglossi Neurons That Project to the Cat Abducens Nucleus Robert F. Spencer,’ Robert J. Wenthold, and Robert Baker3 ‘Department of Anatomy, Medical College of Virginia, Richmond, Virginia 23298, “Laboratory of Neuro-otolaryngology, National Institute of Neurological and Communicative Disorders and Stroke, National Institutes of Health, Bethesda, Maryland 20892, and 3Department of Physiology and Biophysics, New York University Medical Center, New York, New York 10016 The localization and distribution of brain-stem afferent neu- that were labeled autoradiographically by retrograde trans- rons to the cat abducens nucleus has been examined by port of 3H-glycine from the abducens nucleus. high-affinity uptake and retrograde transport of 3H-glycine. Electrophysiological recordings from abducens motoneu- Injections of 3H-glycine selectively labeled (by autoradiog- rons and internuclear neurons revealed a marked reduction raphy) only neurons located predominantly in the ipsilateral in the slow positivity of the orthodromic extracellular poten- medial vestibular and contralateral prepositus hypoglossi tial elicited by ipsilateral vestibular nerve stimulation follow- nuclei, and in the contralateral dorsomedial reticular for- ing systemic administration of strychnine, an antagonist of mation, the latter corresponding to the location of inhibitory glycine. Intracellular recordings demonstrated that the ves- burst neurons. The specificity of uptake and retrograde tibular-evoked disynaptic inhibitory postsynaptic potentials transport of 3H-glycine was indicated by the absence of la- in abducens neurons were effectively blocked by strychnine beling of the dorsomedial medullary reticular neurons ipsi- but were unaffected by picrotoxin, an antagonist of GABA. -

Anatomical Note the Superficial Origin of the Trochlear Nerve with Special
J. Anat. (1990), 170, pp. 199-201 199 With 4 figures Printed in Great Britain Anatomical Note The superficial origin of the trochlear nerve with special reference to its vascular relations K. K. BISARIA*, I. C. PREMSAGARt, P. K. LAKHTAKIAt, R. C. SAXENA* AND S. D. BISARIA§ * Department of Anatomy, t Department of Neurosurgery, $ Department of Orthopaedic Surgery, K. G. Medical College, Lucknow, U.P. and § Department Of Ophthalmology, Vivekananda Polyclinic, Lucknow, U.P. India (Accepted 14 November 1989) INTRODUCTION A full description of the roots of the trochlear nerve is not commonly found in the literature. Gardner, Gray & O'Rahilly (1960), Hamilton (1976), Romanes (1964), Snell (1981), Hollinshead & Rosse (1985) and Williams, Warwick, Dyson & Bannister (1989) do not mention the possibility of two roots of origin of this nerve. Crosby, Humphrey & Lauer (1962) and Truex & Carpenter (1969) have mentioned that the nerve may emerge as a single root while Paturet (1964) and Nathan & Goldhammer (1973) have mentioned the possibility that more than one root may be present. The relations of the trochlear nerve to the adjoining blood vessels have not been described. MATERIALS AND METHODS For the present work 162 trochlear nerves of 90 formalin-fixed brains were studied (in 18 brains only one of the two nerves could be studied as the other was torn during the removal of the brain from the cranial cavity). The origin of the nerve and its relationships with neighbouring blood vessels was noted. RESULTS In all the 90 brains, the trochlear nerves arose in the usual situation. In 111 nerves out of 162, the nerve had a single root; in 51, double rootlets were present, the rootlets being up to 4 mm apart (Fig. -

Brainstem II
Brainstem II Medical Neuroscience Dr. Wiegand Internal Brainstem | Cranial nerve nuclei | Location of selected tracts | Reticular formation Developmental Organization 1 Developmental Organization Sulcus Limitans Developmental Organization FromFrom PritchardPritchard && Alloway:Alloway: Fig.Fig. 4-14-1 2 Cranial Nerve Nuclei Organization | Medial to sulcus limitans z GSE ⇒ SVE ⇒ GVE | Lateral from sulcus limitans z VA ⇒ GSA ⇒ SSA FromFrom PritchardPritchard && Alloway:Alloway: Fig.Fig. 4-44-4 SEN MOT Generalizations | Sensory nuclei lateral to sulcus limitans | Motor nuclei medial to sulcus limitans | Visceral nuclei are on either side of sulcus | Innervation of skeletal muscle (GSE & SVE) most medial | General and special visceral afferent nuclei in same column I, II Cranial Nerves – Telencephalon & Diencephalon | Olfactory – z smell (SVA) | Optic – z vision (SSA) 3 III, IV Cranial Nerves – Mesencephalon | Oculomotor – z extraocular eye muscles (GSE) – oculomotor nucleus z PSNS to eye (GVE) – Edinger-Westphal nucleus | Trochlear – z extraocular muscle (sup. oblique) (GSE) – trochlear nucleus V, VI Cranial Nerves – Metencephalon | Trigeminal – z Masticatory muscles (SVE) – trigeminal motor nucleus z General sensation of the head and face (GSA) – trigeminal complex | Abducens – z extraocular muscle (lat. rectus) (GSE) – abducens nucleus VII Cranial Nerves – Metencephalon | Facial – z Facial expression muscles (SVE) – facial motor nucleus z Glands (submandibular, sublingual & lacrimal) (GVE) – superior salivatory & lacrimal nucleus z Taste (SVA) – rostral solitary nucleus z General sensation of ear (GSA) – trigeminal complex 4 VIII Cranial Nerves – Metencephalon Vestibulocochlear – z Hearing (SSA) – dorsal and ventral cochlear nuclei z Balance (SSA) – vestibular nuclei IX Cranial Nerves – Mylencephalon | Glossopharyngeal z Stylopharyngeus muscle (SVE) – n. ambiguus z PSNS to parotid gland (GVE) – inferior salivatory n. z Taste (SVA) – rostral solitary n. -

Name Mohammad Alsalem
9 AbdulrahmanName Abdllah & Obada Froukh Obada Froukh & Abdulrahman Abdllah Abdulrahman Abdllah & Obada Froukh Mohammad Alsalem 0 The midbrains – Cont. We will study the midbrain on two sections. The first section is at the level of the inferior colliculus, and the second section at the level of the superior colliculus. In the previous lecture, we found out that these colliculi that are found on the posterior aspect of the midbrain, makes up the tectum. 1. Level of inferior colliculus The cavity of the section is the cerebral aqueduct. Anything posterior to the cerebral aqueduct is the tectum, anything anterior to it is the cerebral peduncle (substantia nigra divides the cerebral peduncle to tegmentum (posterior) and crus cerebri (anterior)) Regarding this section, posterior to the cerebral aqueduct are the inferior colliculi. Anterior to the cerebral aqueduct is the nucleus of trochlear nerve (CN4) which is motor. Notice the route of the lower motor neuron of the trochlear nerve. Upon the synapsis of the upper motor neuron of trochlear nerve at this nucleus, lower motor neurons arise and they turn posteriorly around the cerebral aqueduct & the mesencephalic nucleus of trigeminal nerve to emerge from the posterior aspect of the midbrain. (CN4 is the only cranial nerve arising from the posterior aspect of brainstem) Medial longitudinal fasciculus (MLF) is anterolateral to the trochlear nucleus. It connects the motor nuclei of cranial nerves responsible for eyeball movement (CN3, CN4, CN6) with the vestibular nuclei and the upper cervical segments. In this section you can see the decussation of superior cerebellar peduncles, which will eventually form the superior cerebellar peduncle and move towards the cerebellum. -

Lab 3. Pons & Midbrain
Lab 3. Pons & Midbrain Lesion Lessons Lesion 4.1 Anne T. Pasta i) Location ii) Signs/symptoms (Slice of Brain © 993 Univs. of Utah and Washington; E.C. Alvord, Jr., Univ. of Washington) iii) Cause: Lesion 4.2 Colin S. Terase i) Location ii) Signs/symptoms (Slice of Brain © 993 Univs. of Utah and Washington; M.Z. Jones, Michigan St. Univ.) iii) Cause: Medical Neuroscience 4– Pontine Level of the Facial Genu Locate and note the following: Basilar pons – massive ventral structure provides the most obvious change from previous med- ullary levels. Question classic • pontine gray - large nuclear groups in the basilar pons. Is the middle cerebellar peduncle composed – origin of the middle cerebellar peduncle of climbing or mossy • pontocerebellar axons - originate from pontine gray neurons and cross to form the fibers? middle cerebellar peduncle. • corticopontine axons- huge projection that terminates in the basilar pontine gray. • corticospinal tract axons – large bundles of axons surrounded by the basilar pontine gray. – course caudally to form the pyramids in the medulla. Pontine tegmentum • medial lemniscus - has now assumed a “horizontal” position and forms part of the border between the basilar pons and pontine tegmentum. Question classic • central tegmental tract - located just dorsally to the medial lemniscus. What sensory modali- – descends from the midbrain to the inferior olive. ties are carried by the • superior olivary nucleus - pale staining area lateral to the central tegmental tract. medial and lateral – gives rise to the efferent olivocochlear projection to the inner ear. lemnisci? • lateral lemniscus - lateral to the medial lemniscus. – composed of secondary auditory projections from the cochlear nuclei. -
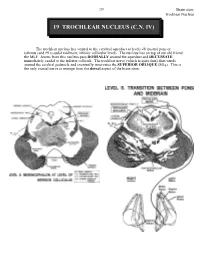
19 Trochlear Nucleus (C.N
299 Brain stem Trochlear Nucleus 19 TROCHLEAR NUCLEUS (C.N. IV) The trochlear nucleus lies ventral to the cerebral aqueduct at levels #8 (rostral pons or isthmus) and #9 (caudal midbrain; inferior collicular level). The nucleus lies on top of our old friend the MLF. Axons from this nucleus pass DORSALLY around the aqueduct and DECUSSATE immediately caudal to the inferior colliculi. The trochlear nerve (which is quite thin) then winds around the cerebral peduncle and eventually innervates the SUPERIOR OBLIQUE (SO4). This is the only cranial nerve to emerge from the dorsal aspect of the brain stem. Brain stem 300 Trochlear nucleus While at first glance it appears that contraction of the superior oblique turns the eye down and out, the rest of the story (Paul Harvey would love this) is slightly more complicated. If you are interested, read on! The vector diagram resolves the arrow R-B into effective components. Vector R-A depresses the eye around the lateral axis. Vector R-C abducts the eye around the vertical axis and intorts (medial rotation) the eye around the anteroposterior axis. Therefore, vector R-B acts to depress, abduct and intort the eye. When the eye is in the primary position, the superior oblique lies medial to the A-P axis of the globe. However, when the eye is adducted, the line of pull of the tendon of the superior oblique is parallel to the A-P axis of the globe. In this position, none of the actions of the muscle are dissipated in the other actions (abduction and intorsion).