Unique Evolution of Vitamin a As an External Pigment In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Traffic.Org/Home/2015/12/4/ Thousands-Of-Birds-Seized-From-East-Java-Port.Html
TRAFFIC IN THE MARKET FOR EXTINCTION REPORT Eastern and Central Java AUGUST 2016 Serene C. L. Chng and James A. Eaton TRAFFIC Report: In The Market for Extinction: Eastern and Central Java 1 TRAFFIC REPORT TRAFFIC, the wild life trade monitoring net work, is the leading non-governmental organization working globally on trade in wild animals and plants in the context of both biodiversity conservation and sustainable development. TRAFFIC is a strategic alliance of WWF and IUCN. Reprod uction of material appearing in this report requires written permission from the publisher. The designations of geographical entities in this publication, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of TRAFFIC or its supporting organizations con cern ing the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views of the authors expressed in this publication are those of the writers and do not necessarily reflect those of TRAFFIC, WWF or IUCN. Published by TRAFFIC. Southeast Asia Regional Office Unit 3-2, 1st Floor, Jalan SS23/11 Taman SEA, 47400 Petaling Jaya Selangor, Malaysia Telephone : (603) 7880 3940 Fax : (603) 7882 0171 Copyright of material published in this report is vested in TRAFFIC. © TRAFFIC 2016. ISBN no: 978-983-3393-50-3 UK Registered Charity No. 1076722. Suggested citation: Chng, S.C.L. and Eaton, J.A. (2016). In the Market for Extinction: Eastern and Central Java. TRAFFIC. Petaling Jaya, Selangor, Malaysia. Front cover photograph: An Oriental Bay Owl Phodilus badius displayed for sale at Malang Bird Market Credit: Heru Cahyono/TRAFFIC IN THE MARKET FOR EXTINCTION Eastern and Central Java Serene C. -

Lambusango Forest Conservation Project Research Proposal 2008
Lambusango Forest Research Project OPERATION WALLACEA Research Report 2011 Compiled by Dr Philip Wheeler (email [email protected]) University of Hull Scarborough Campus, Centre for Environmental and Marine Sciences Contents Contents .................................................................................................................................................. 1 Introduction ............................................................................................................................................ 2 Research Sites ......................................................................................................................................... 3 Research activity 2011 ............................................................................................................................ 3 Monitoring bird communities............................................................................................................. 4 Bat community dynamics ................................................................................................................... 9 Monitoring herpetofauna and small mammal communities ........................................................... 11 Butterfly community dynamics ........................................................................................................ 15 Monitoring anoa and wild pig populations ...................................................................................... 19 Habitat associations and sleeping site characteristics -

Supplement - 2016
Green and black poison dart frog Supplement - 2016 Whitley Wildlife Conservation Trust Paignton Zoo Environmental Park, Living Coasts & Newquay Zoo Supplement - 2016 Index Summary Accounts 4 Figures At a Glance 6 Paignton Zoo Inventory 7 Living Coasts Inventory 21 Newquay Zoo Inventory 25 Scientific Research Projects, Publications and Presentations 35 Awards and Achievements 43 Our Zoo in Numbers 45 Whitley Wildlife Conservation Trust Paignton Zoo Environmental Park, Living Coasts & Newquay Zoo Bornean orang utan Paignton Zoo Inventory Pileated gibbon Paignton Zoo Inventory 1st January 2016 - 31st December 2016 Identification IUCN Status Arrivals Births Did not Other Departures Status Identification IUCN Status Arrivals Births Did not Other Departures Status Status 1/1/16 survive deaths 31/12/16 Status 1/1/16 survive deaths 31/12/16 >30 days >30 days after birth after birth MFU MFU MAMMALIA Callimiconidae Goeldi’s monkey Callimico goeldii VU 5 2 1 2 MONOTREMATA Tachyglossidae Callitrichidae Short-beaked echidna Tachyglossus aculeatus LC 1 1 Pygmy marmoset Callithrix pygmaea LC 5 4 1 DIPROTODONTIA Golden lion tamarin Leontopithecus rosalia EN 3 1 1 1 1 Macropodidae Pied tamarin Saguinus bicolor CR 7 3 3 3 4 Western grey Macropus fuliginosus LC 9 2 1 3 3 Cotton-topped Saguinus oedipus CR 3 3 kangaroo ocydromus tamarin AFROSORICIDA Emperor tamarin Saguinus imperator LC 3 2 1 subgrisescens Tenrecidae Cebidae Lesser hedgehog Echinops telfairi LC 8 4 4 tenrec Squirrel monkey Saimiri sciureus LC 5 5 Giant (tail-less) Tenrec ecaudatus LC 2 2 1 1 White-faced saki Pithecia pithecia LC 4 1 1 2 tenrec monkey CHIROPTERA Black howler monkey Alouatta caraya NT 2 2 1 1 2 Pteropodidae Brown spider monkey Ateles hybridus CR 4 1 3 Rodrigues fruit bat Pteropus rodricensis CR 10 3 7 Brown spider monkey Ateles spp. -

Trip Report 17Th August to 3Rd September 2013
Sulawesi & Halmahera Wallacean Endemics Trip Report 17th August to 3rd September 2013 Lilac Kingfisher by David Hoddinott RBT Sulawesi & Halmahera 2013 Trip Report 2 Trip report compiled by Tour Leader: David Hoddinott Top 10 birds as voted by participants: 1. Standardwing 6. Azure Dollarbird 2. Maleo 7. Moluccan Owlet-nightjar 3. Sulawesi Masked Owl 8. Lilac Kingfisher 4. Ivory-breasted Pitta 9. Red-backed Thrush 5. Mountain Serin 10. Sulawesi Dwarf Kingfisher Tour Summary Surrounded to the north by the Philippines, to the east by New Guinea, the south by Australia and the west by Borneo, the two larger islands of Sulawesi and Halmahera form a significant part of central Indonesia’s nearly 15000 islands. We recorded over 100 endemics of a total trip list of 254 species, thus emphasising that this is certainly one of the endemic hotspots of the world! Our tour started off with an early morning visit to the limestone crags of Karaenta Forest, Sulawesi. Departing Makassar, we set off early to maximise our limited time as we had a flight to catch in the early afternoon. Arriving just as the sun’s first rays hit the treetops, we were soon enjoying wonderful sightings of stunning Grey-sided and Yellow-sided Flowerpeckers. You could actually feel the sense of excitement in the air as we notched up our first endemics. After enjoying our tea and coffee, we then quickly picked up our main target, the rather localised endemic Black-ringed Whit-eye, which showed splendidly as it sat just in front of us gobbling down some ripe fruit. -

Southeast Asia Mega Tour: Singapore/Borneo/Peninsular Malaysia/Halmahera/Sulawesi
Southeast Asia Mega Tour: Singapore/Borneo/Peninsular Malaysia/Halmahera/Sulawesi August 9th-September 30th, 2013 This seven-week tour took us to some of Southeast Asia’s most amazing birding spots, where we racked up some mega targets, saw some amazing scenery, ate some lovely cuisine, and generally had a great time birding. Among some of the fantastic birds we saw were 11 species of pitta, including the endemic Ivory-breasted and Blue-banded Pittas, 27 species of night birds, including the incomparable Satanic Nightjar, Blyth’s, Sunda and Large Frogmouths, and Moluccan Owlet-Nightjar, 14 species of cuckooshrikes, 15 species of kingfishers, and some magical gallinaceous birds like Mountain Peacock- Pheasant, Crested Fireback, and the booming chorus of Argus Pheasant. 13 species of Hornbills were seen, including great looks at Helmeted, White-crowned, Plain-pouched, and Sulawesi. Overall we saw 134 endemic species. Singapore The tour started with some birding around Singapore, and at the Central Catchment Reservoir we started off well with Short-tailed Babbler, Chestnut-bellied Malkoha, Banded Woodpecker, Van Hasselt’s Sunbird, and loads of Pink-necked Green Pigeon. Bukit Batok did well with Straw-headed Bulbul, Common Flameback, and Laced Woodpecker, as well as a particularly obliging group of White- crested Laughingthrush. Borneo We then flew to Borneo, where we began with some local birding along the coast, picking up not only a number of common species of waterbirds but also a mega with White-fronted (Bornean) Falconet. We www.birdingecotours.com [email protected] 2 | TRIP REPORT Southeast Asia Mega Tour: Aug - Sep 2013 ended the day at some rice paddies, where we found Buff-banded Rail and Watercock among several marsh denizens. -
![Explorer Research Article [Tripathi Et Al., 6(3): March, 2015:4304-4316] CODEN (USA): IJPLCP ISSN: 0976-7126 INTERNATIONAL JOURNAL of PHARMACY & LIFE SCIENCES (Int](https://docslib.b-cdn.net/cover/4638/explorer-research-article-tripathi-et-al-6-3-march-2015-4304-4316-coden-usa-ijplcp-issn-0976-7126-international-journal-of-pharmacy-life-sciences-int-1074638.webp)
Explorer Research Article [Tripathi Et Al., 6(3): March, 2015:4304-4316] CODEN (USA): IJPLCP ISSN: 0976-7126 INTERNATIONAL JOURNAL of PHARMACY & LIFE SCIENCES (Int
Explorer Research Article [Tripathi et al., 6(3): March, 2015:4304-4316] CODEN (USA): IJPLCP ISSN: 0976-7126 INTERNATIONAL JOURNAL OF PHARMACY & LIFE SCIENCES (Int. J. of Pharm. Life Sci.) Study on Bird Diversity of Chuhiya Forest, District Rewa, Madhya Pradesh, India Praneeta Tripathi1*, Amit Tiwari2, Shivesh Pratap Singh1 and Shirish Agnihotri3 1, Department of Zoology, Govt. P.G. College, Satna, (MP) - India 2, Department of Zoology, Govt. T.R.S. College, Rewa, (MP) - India 3, Research Officer, Fishermen Welfare and Fisheries Development Department, Bhopal, (MP) - India Abstract One hundred and twenty two species of birds belonging to 19 orders, 53 families and 101 genera were recorded at Chuhiya Forest, Rewa, Madhya Pradesh, India from all the three seasons. Out of these as per IUCN red list status 1 species is Critically Endangered, 3 each are Vulnerable and Near Threatened and rest are under Least concern category. Bird species, Gyps bengalensis, which is comes under Falconiformes order and Accipitridae family are critically endangered. The study area provide diverse habitat in the form of dense forest and agricultural land. Rose- ringed Parakeets, Alexandrine Parakeets, Common Babblers, Common Myna, Jungle Myna, Baya Weavers, House Sparrows, Paddyfield Pipit, White-throated Munia, White-bellied Drongo, House crows, Philippine Crows, Paddyfield Warbler etc. were prominent bird species of the study area, which are adapted to diversified habitat of Chuhiya Forest. Human impacts such as Installation of industrial units, cutting of trees, use of insecticides in agricultural practices are major threats to bird communities. Key-Words: Bird, Chuhiya Forest, IUCN, Endangered Introduction Birds (class-Aves) are feathered, winged, two-legged, Birds are ideal bio-indicators and useful models for warm-blooded, egg-laying vertebrates. -

GRUNDSTEN Sulawesi 0708
Birding South and Central Sulawesi (M. Grundsten, Sweden) 2016 South and Central Sulawesi, July 28th - August 5th 2016 Front cover Forest-dwelling Dwarf Sparrowhawk, Accipiter nanus, Anaso track, Lore Lindu NP (MG). Participants Måns Grundsten [email protected] (compiler and photos (MG)) Mathias Bergström Jonas Nordin, all Stockholm, Sweden. Highlights • Luckily escaping the previous extensive occlusion of Anaso track due to terrorist actions. Anaso track opened up during our staying. • A fruit-eating Tonkean Macaque at Lore Lindu. • Great views of hunting Eastern Grass Owls over paddies around Wuasa on three different evenings. • Seeing a canopy-perched Sombre Pigeon above the pass at Anaso track. • Flocks of Malias and a few Sulawesi Thrushes. • No less than three different Blue-faced Parrotfinches. • Purple-bearded Bee-eaters along Anaso track. • Finding Javan Plover at Palu salt pans, to our knowledge a significant range extension, previously known from Sulawesi mainly in Makassar-area. • Sulawesi Hornbill at Paneki valley. • Sulawesi Streaked Flycatcher at Paneki valley, a possibly new location for this recently described species. • Two days at Gunung Lompobattang in the south: Super-endemic Lompobattang Flycatcher, Black-ringed White-eye, and not-so-easy diminutive Pygmy Hanging Parrot. Logistics With limited time available this was a dedicated trip to Central and Southern Sulawesi. Jonas and Mathias had the opportunity to extend the trip for another week in the North while I had to return home. The trip was arranged with help from Nurlin at Palu-based Malia Tours ([email protected]). Originally we had planned to have a full board trip to Lore Lindu and also include seldom-visited Saluki in the remote western parts of Lore Lindu, a lower altitude part of Lore Lindu where Maleo occurs. -

The Avifauna of Lambusango Forest Reserve, Buton Island, South-East Sulawesi, with Additional Sightings from Southern Buton
FORKTAIL 28 (2012): 107–112 The avifauna of Lambusango Forest Reserve, Buton Island, south-east Sulawesi, with additional sightings from southern Buton T. E. MARTIN, D. J. KELLY, N. T. KEOGH, D. HERIYADI, H. A. SINGER & G. A. BLACKBURN Lambusango Forest Reserve occupies a large area of south-central Buton, the largest attendant island of Sulawesi, Indonesia. Buton is located off Sulawesi’s south-eastern peninsula and remains poorly known ornithologically. Bird surveys were undertaken in the reserve over eight eight-week long research seasons between June and August in 1999, 2001–2003, 2005, and 2008–2010. Variable radius circular- plot point counts were the primary census method, conducted as part of a long-term biodiversity monitoring programme in the reserve, although data were also collected from 840 mist-netting hours and approximately 2,560 hours of observational data. In total, 79 species were detected in the reserve, including 37 regional endemics (46.8% of the total avifaunal community) and four species considered by the IUCN to be globally threatened or Near Threatened. Additionally, a further 60 species (including two more Near Threatened species) were recorded in various habitats around southern Buton that were not detected in Lambusango Reserve, giving a total of 139 species records for the island. We believe that 51 of these species represent previously unpublished records for Buton. We present here a full account of our records from Lambusango Reserve and southern Buton, with additional details provided for threatened and Near Threatened species and new records of endemics. INTRODUCTION Lambusango Forest Reserve (5°10’–5°24’S 122°43’–123°07’E) is a 65,000 ha expanse of uninhabited tropical monsoon forest, Buton (formerly referred to as Butung) is the largest of Sulawesi’s encompassing much of south-central Buton. -

Conservation of Breeding Colonies of the Bank Myna, Acridotheres Ginginianus (Latham 1790), in Chapai Nawabganj, Bangladesh
Univ. j. zool. Rajshahi Univ. Vol. 30, 2011 pp. 49-53 ISSN 1023-6104 http://journals.sfu.ca/bd/index.php/UJZRU © Rajshahi University Zoological Society Conservation of breeding colonies of the bank myna, Acridotheres ginginianus (Latham 1790), in Chapai Nawabganj, Bangladesh M Farid Ahsan and M Tarik Kabir Department of Zoology, University of Chittagong, Chittagong, Bangladesh Abstact: A study on the conservation on breeding colonies of bank myna, Acridotheres ginginianus (Latham 1790), was conducted in Chapai Nawabganj district of Bangladesh during a period from June 2007 to October 2009. The total population in the breeding colonies was estimated as 4,452. The preferable breeding habitats of bird are bank of the river, soil heap/ditches of brickfield and holes of culvert. Environmental calamities such as bank erosion and flooding, drought and rain affect the breeding colonies. Human settlement near breeding habitats, hunting, collecting eggs and nestlings by children, and use of nets to prevent nesting at bank of the river are the main human impacts on the breeding colonies. Major threats are flood, children-fun, predators and human for its meat. Conservation awareness for this species have depicted in this paper. Key words: Conservation, breeding colonies, bank myna, Acridotheres ginginianus, Chapai Nawabganj, Bangladesh. example, Grimmett et al. (1998), Inskipp et al. Introduction (1996), Sibley & Monroe (1993, 1990), Sibley & Conservation biology is the new multidisciplinary Ahlquist (1990), Ripley (1982), Husain (2003, science that -

Sturnus Roseus
Report under the Article 12 of the Birds Directive European Environment Agency Period 2008-2012 European Topic Centre on Biological Diversity Sturnus roseus Annex I No International action plan No Rosy Starling, Sturnus roseus, is a species of passerine bird in the starling family found in heathland and shrub and unvegetated or sparsely vegetated land ecosystems. Sturnus roseus has a breeding population size of 260-32300 pairs and a breeding range size of 3700 square kilometres in the EU27. The breeding population trend in the EU27 is Unknown in the short term and Unknown in the long term. The EU population status for Sturnus roseus is Unknown, as the data reported were not sufficient to assess the population status of the species. Page 1 Sturnus roseus Report under the Article 12 of the Birds Directive Assessment of status at the European level Breeding Breeding range Winter population Winter Breeding population trend Range trend trend Population population population size area status Short Long Short Long size Short Long term term term term term term 260 - 32300 p x x 3700 Unknown See the endnotes for more informationi Page 2 Sturnus roseus Report under the Article 12 of the Birds Directive Page 3 Sturnus roseus Report under the Article 12 of the Birds Directive Trends at the Member State level Breeding Breeding range Winter population Winter % in Breeding population trend Range trend trend MS/Ter. population EU27 population size area Short Long Short Long size Short Long term term term term term term BG 53.3 10 - 7300 p F F 1600 F F GR RO 46.7 250 - 24000 p x x 2100 x x See the endnotes for more informationii Page 4 Sturnus roseus Report under the Article 12 of the Birds Directive Page 5 Sturnus roseus Report under the Article 12 of the Birds Directive Short-term winter population trend was not reported for this species. -
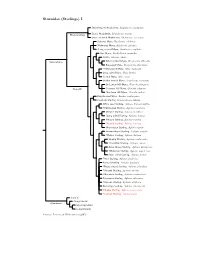
Sturnidae Tree, Part 1
Sturnidae (Starlings) I Stripe-headed Rhabdornis, Rhabdornis mystacalis Grand Rhabdornis, Rhabdornis grandis Rhabdornithini Stripe-breasted Rhabdornis, Rhabdornis inornatus Sulawesi Myna, Basilornis celebensis ?Helmeted Myna, Basilornis galeatus ?Long-crested Myna, Basilornis corythaix Apo Myna, Goodfellowia mirandus Coleto, Sarcops calvus Graculinae White-necked Myna, Streptocitta albicollis Bare-eyed Myna, Streptocitta albertinae ?Yellow-faced Myna, Mino dumontii Long-tailed Myna, Mino kreffti Golden Myna, Mino anais Golden-crested Myna, Ampeliceps coronatus Sri Lankan Hill-Myna, Gracula ptilogenys Graculini Common Hill Myna, Gracula religiosa ?Southern Hill Myna, Gracula indica Fiery-browed Myna, Enodes erythrophris Grosbeak Starling, Scissirostrum dubium White-eyed Starling, Aplonis brunneicapillus ?Yellow-eyed Starling, Aplonis mystacea Metallic Starling, Aplonis metallica ?Long-tailed Starling, Aplonis magna Pohnpei Starling, Aplonis pelzelni ?Kosrae Starling, Aplonis corvina Micronesian Starling, Aplonis opaca Brown-winged Starling, Aplonis grandis ?Makira Starling, Aplonis dichroa Singing Starling, Aplonis cantoroides ?Tanimbar Starling, Aplonis crassa Asian Glossy Starling, Aplonis panayensis ?Moluccan Starling, Aplonis mysolensis Short-tailed Starling, Aplonis minor ?Atoll Starling, Aplonis feadensis Rennell Starling, Aplonis insularis ?Rusty-winged Starling, Aplonis zelandica ?Striated Starling, Aplonis striata ?Mountain Starling, Aplonis santovestris Polynesian Starling, Aplonis tabuensis ?Samoan Starling, Aplonis atrifusca Rarotonga Starling, Aplonis cinerascens ?Mauke Starling, Aplonis mavornata ?Tasman Starling, Aplonis fusca Sturnini Cinnyricinclini Sturninae Onychognathini Lamprotornini Sources: Lovette and Rubenstein (2007).. -

Migratory Birds of Ladakh a Brief Long Distance Continental Migration
WORLD'S MIGRATORY BIRDS DAY 08 MAY, 2021 B R O W N H E A D E D G U L L MIGRATORY BIRDS OF LADAKH A BRIEF LONG DISTANCE CONTINENTAL MIGRATION the Arctic Ocean and the Indian Ocean, and comprises several migration routes of waterbirds. It also touches “West Asian- East African Flyway”. Presence of number of high-altitude wetlands (>2500 m amsl altitude) with thin human population makes Ladakh a suitable habitat for migration and breeding of continental birds, including wetlands of very big size (e.g., Pangong Tso, Tso Moriri, Tso Kar, etc.). C O M M O N S A N D P I P E R Ladakh provides a vast habitat for the water birds through its complex Ladakh landscape has significance network of wetlands including two being located at the conjunction of most important wetlands (Tso Moriri, four zoogeographic zones of the world Tso Kar) which have been designated (Palearctic, Oriental, Sino-Japanese and as Ramsar sites. Sahara-Arabian). In India, Ladakh landscape falls in Trans-Himalayan Nearly 89 bird species (long distance biogeographic zone and two provinces migrants) either breed or roost in (Ladakh Mountains, 1A) and (Tibetan Ladakh, and most of them (59) are Plateau, 1B). “Summer Migrants”, those have their breeding grounds here. Trans-Himalayan Ladakh is an integral part of the "Central Asian Flyway" of migratory birds which a large part of the globe (Asia and Europe) between Ladakh also hosts 25 bird species, during their migration along the Central Asian Flyway, as “Passage Migrants” which roost in the region.