Accepted Manuscript
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

B Commission Regulation (Eu)
02010R0037 — EN — 29.09.2018 — 035.001 — 1 This text is meant purely as a documentation tool and has no legal effect. The Union's institutions do not assume any liability for its contents. The authentic versions of the relevant acts, including their preambles, are those published in the Official Journal of the European Union and available in EUR-Lex. Those official texts are directly accessible through the links embedded in this document ►B COMMISSION REGULATION (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin (Text with EEA relevance) (OJ L 15, 20.1.2010, p. 1) Amended by: Official Journal No page date ►M1 Commission Regulation (EU) No 758/2010 of 24 August 2010 L 223 37 25.8.2010 ►M2 Commission Regulation (EU) No 759/2010 of 24 August 2010 L 223 39 25.8.2010 ►M3 Commission Regulation (EU) No 761/2010 of 25 August 2010 L 224 1 26.8.2010 ►M4 Commission Regulation (EU) No 890/2010 of 8 October 2010 L 266 1 9.10.2010 ►M5 Commission Regulation (EU) No 914/2010 of 12 October 2010 L 269 5 13.10.2010 ►M6 Commission Regulation (EU) No 362/2011 of 13 April 2011 L 100 26 14.4.2011 ►M7 Commission Regulation (EU) No 363/2011 of 13 April 2011 L 100 28 14.4.2011 ►M8 Commission Implementing Regulation (EU) No 84/2012 of 1 L 30 1 2.2.2012 February 2012 ►M9 Commission Implementing Regulation (EU) No 85/2012 of 1 L 30 4 2.2.2012 February 2012 ►M10 Commission Implementing Regulation (EU) No 86/2012 of 1 L 30 6 2.2.2012 February 2012 ►M11 Commission -

Report Name:Ukraine's Mrls for Veterinary Drugs
Voluntary Report – Voluntary - Public Distribution Date: November 05,2020 Report Number: UP2020-0051 Report Name: Ukraine's MRLs for Veterinary Drugs Country: Ukraine Post: Kyiv Report Category: FAIRS Subject Report Prepared By: Oleksandr Tarassevych Approved By: Robin Gray Report Highlights: Ukraine adopted several maximum residue levels (MRLs) for veterinary drugs, coccidiostats and histomonostats in food products of animal origin. Ukraine also adopted a list of drugs residues that are not allowed in food products. THIS REPORT CONTAINS ASSESSMENTS OF COMMODITY AND TRADE ISSUES MADE BY USDA STAFF AND NOT NECESSARILY STATEMENTS OF OFFICIAL U.S. GOVERNMENT POLICY The Office of Agricultural Affairs of USDA/Foreign Agricultural Service in Kyiv, Ukraine prepared this report for U.S. exporters of domestic food and agricultural products. While every possible care was taken in the preparation of this report, information provided may not be completely accurate either because policies have changed since the time this report was written, or because clear and consistent information about these policies was not available. It is highly recommended U.S. exporters verify the full set of import requirements with their foreign customers, who are normally best equipped to research such matters with local authorities, before any goods are shipped. This FAIRS Subject Report accompanies other reports on Maximum, Residue Limits established by Ukraine in 2020. Related reports could be found under the following links: 1.) Ukraine's MRLs for Microbiological Contaminants_Kyiv_Ukraine_04-27-2020 2.) Ukraine's MRLs for Certain Contaminants_Kyiv_Ukraine_03-06-2020 Food Products of animal origin and/or ingredients of animal origin are not permitted in the Ukrainian market if they contain certain veterinary drugs residues in excess of the maximum residue levels established in Tables 1 and 2. -

Environmental Effects of Chemicals Used Against Salmon Lice
STRANDBÚNAÐUR 2018 Grand Hótel Reykjavík, 19. – 20. mars 2018 Renée Katrin Bechmann Environmental effects of chemicals used against salmon lice 21 March 2018 In aquaculture, pesticides are used against parasitic salmon lice to protect the health of farmed and wild Atlantic salmon. - How can this use of pesticides as medicine affect our coastal marine environment? Economic consequences Costs billions for the aquaculture industry Salmon lice and the war against lice Environmental concequences Animal welfare for farmed and wild salmon How to get rid of lice Risk for reduced stocks of wild salmon Kill them with chemicals Animal welfare and overfishing of cleaner fish Use cleaner fish and other non-chemical methods Risk for non-target crustaceans, and the rest of Protect the salmon from lice in (semi-)closed cages the coastal ecosystem The perfect anti-salmon lice medicine Low toxicity to: Humans High toxicity to: Salmon lice Salmon = target crustaceans Must eat the chemical or swim in a solution Fast depuration after treatment of the fish The environment including non-target crustaceans Must die! Fast degradation Low bioavailability … and not develop resistance Low toxicity kg used per year (log scale) 100000000 10000000 1000000 100000 10000 1000 100 10 1 1981 salmon lice 1981 usedChemicals against 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 - 2010 2017 2011 2012 2013 2014 2015 2016 2017 peroxide Hydrogen benzoate Emamectin Deltamethrin -

Chemotherapeutants Against Salmon Lice Lepeophtheirus Salmonis – Screening of Efficacy
Chemotherapeutants against salmon lice Lepeophtheirus salmonis – screening of efficacy Stian Mørch Aaen Thesis for the degree of Philosophiae Doctor Department of Food Safety and Infection Biology Faculty of Veterinary Medicine and Biosciences Norwegian University of Life Sciences Adamstuen 2016 1 2 TABLE OF CONTENTS Acknowledgments 5 Acronyms/terminology 7 List of papers 8 Summary 9 Sammendrag 9 1 Introduction 11 1.1 Salmon farming in an international perspective; industrial challenges 11 1.2 Salmon lice 12 1.2.1 History and geographic distribution 12 1.2.2 Salmon lice life cycle 14 1.2.3 Pathology caused by salmon lice 16 1.2.4 Salmon lice cultivation in the lab 16 1.3 Approaches to combat sea lice 17 1.3.1 Medicinal interference: antiparasitic chemotherapeutants 17 1.3.2 Resistance in sea lice against chemotherapeutants 19 1.3.3 Non-medicinal intervention: examples 22 1.3.3.1 Physical barriers 23 1.3.3.2 Optical and acoustic control measures 23 1.3.3.3 Functional feeds, vaccine, breeding 24 1.3.3.4 Biological de-lousing: cleaner fish and freshwater 24 1.3.3.5 Physical removal 24 1.3.3.6 Fallowing and geographical zones 25 1.4 Rationale 25 2 Aims 26 3 Materials and methods 26 3.1 Materials 26 3.1.1 Salmon lice 26 3.1.2 Fish – Atlantic salmon 26 3.1.3 Water 27 3.1.3 Medicinal compounds 27 3.1.4 Dissolvents 29 3.2 Methods 29 3.2.1 Hatching assays with egg strings 29 3.2.2 Survival assays with nauplii 29 3.2.3 Bioassays with preadults 30 3.2.4 Statistical analysis 31 4 Summary of papers, I-IV 32 5 Discussion 35 5.1 Novel methods for medicine screening 35 5.2 Industrial innovation in aquaculture and pharmaceutical companies 35 5.3 Administration routes of medicinal compounds to fish 36 5.4 Mixing and bioavailability of medicinal products in seawater 37 5.5 Biochemical targets in L. -

Accreditation No: 15028
SCOPE OF ACCREDITATION - No. 15028 Page 1 of 4 ACCREDITATION NO: 15028 Eurofins Agroscience Testing Pty Ltd Chemical Testing Laboratory Unit F6, Building F 16 Mars Road LANE COVE NSW 2066 CONTACT: Ms K Ford PHONE: (02) 9900 8442 FAX: MOBILE: 0421 609 757 EMAIL: [email protected] WEB SITE: www.eurofins.com.au FACILITIES: Public testing service This facility complies with the requirements of ISO/IEC 17025:2005 7.37 Plastics .11 Chemical analyses Analysis of polyethylene products listed as determination(s) by technique(s) using method(s) - Bifenthrin by GC using in-house AATM-P94 7.52 Residues and contaminants in foods and agricultural materials .02 Pesticides Analysis of animal feeds, animal tissues (fat, kidney, liver, muscle), cereals, fruit, grains, juices/juice concentrate, pasture, pulses, oil seeds, vegetables listed as determination(s) by technique(s) using method(s) - Fungicides: Azoxystrobin; benalaxyl; benomyl; bitertanol; boscalid; bupirimate; carbendazim; carboxin; cyproconazole; difenoconazole; dimethomorph; epoxiconazole; fenarimol; fenbuconazole; fenhexamid; fenpyroximate; fludioxinil; fluquinconazole; flusilazole; flutriafol; hexaconazole; imazilil; ipconazole; iprodione; kresoxim-methyl; mancozeb; maneb; metalaxyl; metiram; myclobutanil; oxadixyl; penconazole; procymidone; propiconazole; propineb; pyraclostrobin; tebuconazole; tetraconazole; thiabendazole; thiram; triadimefon; triadimenol; tridemorph; trifloxystrobin; vinclozolin; zineb; ziram Herbicides: Ametryn; atrazine; carfentrazone ethyl; clethodim; clomazone; clopyralid; -

Recent Advances on Detection of Insecticides Using Optical Sensors
sensors Review Recent Advances on Detection of Insecticides Using Optical Sensors Nurul Illya Muhamad Fauzi 1, Yap Wing Fen 1,2,*, Nur Alia Sheh Omar 1,2 and Hazwani Suhaila Hashim 2 1 Functional Devices Laboratory, Institute of Advanced Technology, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia; [email protected] (N.I.M.F.); [email protected] (N.A.S.O.) 2 Department of Physics, Faculty of Science, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia; [email protected] * Correspondence: [email protected] Abstract: Insecticides are enormously important to industry requirements and market demands in agriculture. Despite their usefulness, these insecticides can pose a dangerous risk to the safety of food, environment and all living things through various mechanisms of action. Concern about the environmental impact of repeated use of insecticides has prompted many researchers to develop rapid, economical, uncomplicated and user-friendly analytical method for the detection of insecticides. In this regards, optical sensors are considered as favorable methods for insecticides analysis because of their special features including rapid detection time, low cost, easy to use and high selectivity and sensitivity. In this review, current progresses of incorporation between recognition elements and optical sensors for insecticide detection are discussed and evaluated well, by categorizing it based on insecticide chemical classes, including the range of detection and limit of detection. Additionally, this review aims to provide powerful insights to researchers for the future development of optical sensors in the detection of insecticides. Citation: Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Hashim, H.S. Recent Keywords: insecticides; optical sensor; recognition element Advances on Detection of Insecticides Using Optical Sensors. -

A Review of Potential Environmental Risks Associated with the Use Of
Canadian Science Advisory Secretariat (CSAS) Research Document 2013/050 National Capital Region A review of potential environmental risks associated with the use of pesticides to treat Atlantic salmon against infestations of sea lice in southwest New Brunswick, Canada Dr. Les Burridge Fisheries and Oceans Canada, St. Andrews Biological Station, 531 Brandy Cove Road, St. Andrews, NB, E5B 2L9 August 2013 Foreword This series documents the scientific basis for the evaluation of aquatic resources and ecosystems in Canada. As such, it addresses the issues of the day in the time frames required and the documents it contains are not intended as definitive statements on the subjects addressed but rather as progress reports on ongoing investigations. Research documents are produced in the official language in which they are provided to the Secretariat. Published by: Fisheries and Oceans Canada Canadian Science Advisory Secretariat 200 Kent Street Ottawa ON K1A 0E6 http://www.dfo-mpo.gc.ca/csas-sccs/ [email protected] © Her Majesty the Queen in Right of Canada, 2013 ISSN 1919-5044 Correct citation for this publication: Burridge, L. 2013. A review of potential environmental risks associated with the use of pesticides to treat Atlantic salmon against infestations of sea lice in southwest New Brunswick, Canada. DFO Can. Sci. Advis. Sec. Res. Doc. 2013/050. iv + 25 p. ii TABLE OF CONTENTS ABSTRACT ............................................................................................................................... III RÉSUMÉ ................................................................................................................................. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

Anti-Sea Lice Agents in Norwegian Aquaculture
Anti-sea lice agents in Norwegian aquaculture surveillance, treatment trends and possible implications for food safety Hannisdal, Rita; Nøstbakken, Ole Jakob; Hove, Helge; Madsen, Lise; Horsberg, Tor Einar; Lunestad, Bjørn Tore Published in: Aquaculture DOI: 10.1016/j.aquaculture.2020.735044 Publication date: 2020 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Hannisdal, R., Nøstbakken, O. J., Hove, H., Madsen, L., Horsberg, T. E., & Lunestad, B. T. (2020). Anti-sea lice agents in Norwegian aquaculture: surveillance, treatment trends and possible implications for food safety. Aquaculture, 521, [735044]. https://doi.org/10.1016/j.aquaculture.2020.735044 Download date: 09. apr.. 2020 Aquaculture 521 (2020) 735044 Contents lists available at ScienceDirect Aquaculture journal homepage: www.elsevier.com/locate/aquaculture Anti-sea lice agents in Norwegian aquaculture; surveillance, treatment T trends and possible implications for food safety ⁎ Rita Hannisdala, , Ole Jakob Nøstbakkena, Helge Hovea, Lise Madsena,c, Tor Einar Horsbergb, Bjørn Tore Lunestada a Institute of Marine Research (IMR), Bergen, Norway. b Norwegian University of Life Sciences (NMBU), Norway. c Department of Biology, University of Copenhagen, Copenhagen, Denmark ARTICLE INFO ABSTRACT Keywords: Sea lice are a major challenge for the Norwegian aquaculture, and to cope with sea lice infections, several Aquaculture physical, biological and chemical treatments are applied. This study presents data on the use of anti-sea lice Salmon agents for Norwegian farmed fish from 1992 to 2017, and results from the surveillance of residues of suchagents Surveillance in samples collected from 2002 to 2017. In the period 2002–2007 the use of anti-sea lice agents included Veterinary drug emamectin, cypermethrin and deltamethrin. -

Silent Spring of the Sea
Silent Spring of the Sea Don Staniford achel Carson’s classic book Silent Spring (1962) first raised public aware- Rness of the environmental risks of human-made chemicals such as DDT, PCBs and dioxins. In her less well-known previous books, The Sea Around Us (1951)and The Edge of the Sea (1955), she marvelled at the wonders of the ocean.1 A half-century later the sea has become a sink for a Molotov cocktail of cancer-causing chemicals and contaminants. Rivers carry pollutants from agricultural runoff, industrial discharges, hazardous wastes and human sewage. Pesticides used in terrestrial farming systems are so noxious that they have wiped out whole river systems. Hormone-disrupting compounds can even change the sex of fish. The sea, some seven-tenths of the earth’s surface,2 is the world’s waste depository—our toxic toilet. We have polluted our marine environment to such an extent that we are literally reaping the consequences via the bioaccumulation of contaminants up the food chain. In the Northern Hemisphere hotspots of chemical con- tamination—the Baltic, Mediterranean and North seas—wild fish from European waters are now eight times more contaminated than those from the South Pacific.3 As well, the World Health Organization has issued a caution acknowl- edging elevated pollution levels in farmed fish, saying “the risk of consuming contaminated fish must be weighed in view of the beneficial nutritive effects of fish.”4 A paper published in January 2004 in the prestigious scientific jour- nal Science revealed that farmed salmon was contaminated with fourteen can- cer-causing chemicals including DDT, PCBs, dieldrin, dioxins, chlordane, toxaphene, lindane and hexachlorobenzene. -

Reflection Paper on Resistance in Ectoparasites
1 13 September 2018 2 EMA/CVMP/EWP/310225/2014 3 Committee for Medicinal Products for Veterinary Use (CVMP) 4 Reflection paper on resistance in ectoparasites 5 Draft Draft agreed by Efficacy Working Party (EWP-V) May 2018 Adopted by CVMP for release for consultation September 2018 Start of public consultation 21 September 2018 End of consultation (deadline for comments) 31 August 2019 6 Comments should be provided using this template. The completed comments form should be sent to [email protected] 7 Keywords Ectoparasites, resistance to ectoparasiticides 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. Reproduction is authorised provided the source is acknowledged. 8 Reflection paper on resistance in ectoparasites 9 Table of contents 10 1. Introduction ....................................................................................................................... 4 11 2. Definition of resistance ...................................................................................................... 4 12 3. Current state of ectoparasite resistance ............................................................................ 4 13 3.1. Ticks .............................................................................................................................. 4 14 3.2. Mites ............................................................................................................................. -
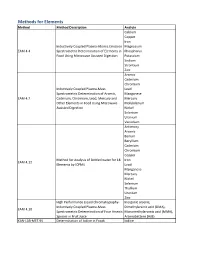
Method Description
Methods for Elements Method Method Description Analyte Calcium Copper Iron Inductively Coupled Plasma-Atomic Emission Magnesium EAM 4.4 Spectrometric Determination of Elements in Phosphorus Food Using Microwave Assisted Digestion Potassium Sodium Strontium Zinc Arsenic Cadmium Chromium Inductively Coupled Plasma-Mass Lead Spectrometric Determination of Arsenic, Manganese EAM 4.7 Cadmium, Chromium, Lead, Mercury and Mercury Other Elements in Food Using Microwave Molybdenum Assisted Digestion Nickel Selenium Uranium Vanadium Antimony Arsenic Barium Beryllium Cadmium Chromium Copper Method for Analysis of Bottled water for 18 Iron EAM 4.12 Elements by ICPMS Lead Manganese Mercury Nickel Selenium Thallium Uranium Zinc High Performance Liquid Chromatography- Inorganic arsenic, Inductively Coupled Plasma-Mass Dimethylarsinic acid (DMA), EAM 4.10 Spectrometric Determination of Four Arsenic Monomethylarsonic acid (MMA), Species in Fruit Juice Arsenobetaine (AsB) KAN-LAB-MET.95 Determination of Iodine in Foods Iodine Methods for Radionuclides Method Method Description Analyte Determination of Strontium-90 in Foods by WEAC.RN.METHOD.2.0 Strontium-90 Internal Gas-Flow Proportional Counting Americium-241 Cesium-134 Cesium-137 Determination of Gamma-Ray Emitting Cobalt-60 WEAC.RN.METHOD.3.0 Radionuclides in Foods by High-Purity Potassium-40 Germanium Spectrometry Radium-226 Ruthenium-103 Ruthenium-106 Thorium-232 Methods for Pesticides/Industrial Chemicals Method Method Description Analyte Extraction Method: Analysis of Pesticides KAN-LAB-PES.53 and