Effects of Soil Water Deficit on Three Tree Species of the Arid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conocarpus Erectus
Conocarpus erectus (Button Mangrove, Green Buttonwood) Button mangrove is a broadleaf evergreen trees which can withstand drought, salt, heat and high winds.The fruit looks like a dried raspberry or a pine cone. Its flaky brown bark is very attractive. Throughout the year, greenish-white and purple flowers are produced, but they are not noticeable. Due to the high tolerance of heat and drought it is used a lot in hot and arid climate as hedge, street tree or windbreak. Landscape Information French Name: Chêne Guadeloupe ﺩﻣﺲ ﻗﺎﺋﻢ :Arabic Name Pronounciation: kawn-oh-KAR-pus ee-RECK- tus Plant Type: Tree Origin: Florida and the West Indies Heat Zones: 9, 10, 11, 12, 14, 15, 16 Hardiness Zones: 10, 11, 12, 13 Uses: Screen, Hedge, Bonsai, Specimen, Container, Shade, Windbreak, Pollution Tolerant / Urban, Reclamation Size/Shape Growth Rate: Moderate Plant Image Tree Shape: Spreading, Vase Canopy Symmetry: Symmetrical Canopy Density: Medium Canopy Texture: Fine Height at Maturity: 8 to 15 m Spread at Maturity: 8 to 10 meters Conocarpus erectus (Button Mangrove, Green Buttonwood) Botanical Description Foliage Leaf Arrangement: Alternate Leaf Venation: Pinnate Leaf Persistance: Evergreen Leaf Type: Simple Leaf Blade: 5 - 10 cm Leaf Shape: Lanceolate Leaf Margins: Entire Leaf Textures: Glossy, Fine Leaf Scent: No Fragance Color(growing season): Green Color(changing season): Green Flower Image Flower Flower Showiness: False Flower Color: Green, White Seasons: Year Round Trunk Trunk Susceptibility to Breakage: Generally resists breakage Number of -

Conocarpus Erectus" Plant As Biomonitoring of Soil and Air Pollution in Ahwaz Region
Middle-East Journal of Scientific Research 13 (10): 1319-1324, 2013 ISSN 1990-9233 © IDOSI Publications, 2013 DOI: 10.5829/idosi.mejsr.2013.13.10.1182 Evaluation of "Conocarpus erectus" Plant as Biomonitoring of Soil and Air Pollution in Ahwaz Region 12Ali Gholami, Amir Hossein Davami, 3Ebrahim Panahpour and 4Hossein Amini 1,3Department of Soil Science, Science and Research Branch, Islamic Azad University, Khouzestan, Iran 2Department of Environmental Management, Science and Research Branch, Islamic Azad University, Khouzestan, Iran 4Department of Soil Science, Islamic Azad University, Khorasgan Branch, Isfahan, Iran Abstract: Effects of soil and atmosphere pollution on some heavy metals (Fe, Zn, Pb, Cu, Mn and Cd) concentration in Button-tree (Conocarpus erectus) leaves were studied in the city of Ahwaz (Khouzestan, Iran). Samples were collected from four sampling sites representing area of high traffic density, area future away from traffic and Industrial area. Samples were collected in two stages (May and October) in 2011 for chemical analysis. Samples from village near the city also analyzed for comparison. Based on the results, the stages of leaf sampling did not showed any significant effect on the concentration of the measured heavy metals in leaf samples. Chemical analysis of soil samples at depth of 0-10cm showed that concentration of most of these elements was lower than the maximum recommended levels. Concentrations of measured heavy metals in washed leaves were lower than those of unwashed leaves of Conocarpus and different was significant. In spite of that, there was no significant correlation between the concentrations of heavy metals in washed leaves and soil samples. -

TAXON:Conocarpus Erectus L. SCORE:5.0 RATING:Evaluate
TAXON: Conocarpus erectus L. SCORE: 5.0 RATING: Evaluate Taxon: Conocarpus erectus L. Family: Combretaceae Common Name(s): button mangrove Synonym(s): Conocarpus acutifolius Willd. ex Schult. buttonwood Conocarpus procumbens L. Sea mulberry Assessor: Chuck Chimera Status: Assessor Approved End Date: 30 Jul 2018 WRA Score: 5.0 Designation: EVALUATE Rating: Evaluate Keywords: Tropical Tree, Naturalized, Coastal, Pure Stands, Water-Dispersed Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 n Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 n outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 y 302 Garden/amenity/disturbance weed 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) n 305 Congeneric weed n=0, y = 1*multiplier (see Appendix 2) n 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals 405 Toxic to animals y=1, n=0 n 406 Host for recognized pests and pathogens 407 Causes allergies or is otherwise toxic to humans y=1, n=0 n 408 Creates a fire hazard in natural ecosystems y=1, n=0 n 409 Is a shade tolerant plant at some stage of its life cycle y=1, n=0 n Creation Date: 30 Jul 2018 (Conocarpus erectus L.) Page 1 of 17 TAXON: Conocarpus erectus L. -

A Caenorhabditis Elegans Model for Discovery of Novel Anti-Infectives
fmicb-07-01956 November 30, 2016 Time: 12:40 # 1 REVIEW published: 02 December 2016 doi: 10.3389/fmicb.2016.01956 Beyond Traditional Antimicrobials: A Caenorhabditis elegans Model for Discovery of Novel Anti-infectives Cin Kong†, Su-Anne Eng, Mei-Perng Lim and Sheila Nathan* School of Biosciences and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, Bangi, Malaysia The spread of antibiotic resistance amongst bacterial pathogens has led to an urgent need for new antimicrobial compounds with novel modes of action that minimize the potential for drug resistance. To date, the development of new antimicrobial drugs is still lagging far behind the rising demand, partly owing to the absence of an effective screening platform. Over the last decade, the nematode Caenorhabditis elegans Edited by: Luis Cláudio Nascimento Da Silva, has been incorporated as a whole animal screening platform for antimicrobials. This CEUMA University, Brazil development is taking advantage of the vast knowledge on worm physiology and how it Reviewed by: interacts with bacterial and fungal pathogens. In addition to allowing for in vivo selection Osmar Nascimento Silva, of compounds with promising anti-microbial properties, the whole animal C. elegans Universidade Católica Dom Bosco, Brazil screening system has also permitted the discovery of novel compounds targeting Francesco Imperi, infection processes that only manifest during the course of pathogen infection of the Sapienza University of Rome, Italy host. Another advantage of using C. elegans in the search for new antimicrobials is that *Correspondence: Sheila Nathan the worm itself is a source of potential antimicrobial effectors which constitute part of its [email protected] immune defense response to thwart infections. -

Population Structure of the Lower Keys Marsh Rabbit As Determined by Mitochondrial DNA Analysis
Management and Conservation Note Population Structure of the Lower Keys Marsh Rabbit as Determined by Mitochondrial DNA Analysis AMANDA L. CROUSE, College of Veterinary Medicine, Texas A&M University, College Station, TX 77843-4461, USA RODNEY L. HONEYCUTT, Natural Science Division, Pepperdine University, Malibu, CA 90263-4321, USA ROBERT A. MCCLEERY,1 Department of Wildlife and Fisheries Sciences, Texas A&M University, College Station, TX 77843-2258, USA CRAIG A. FAULHABER, Department of Wildland Resources, Utah State University, Logan, UT 84322-5230, USA NEIL D. PERRY, Utah Division of Wildlife Resources, Cedar City, UT 84270-0606, USA ROEL R. LOPEZ, Department of Wildlife and Fisheries Sciences, Texas A&M University, College Station, TX 77843-2258, USA ABSTRACT We used nucleotide sequence data from a mitochondrial DNA fragment to characterize variation within the endangered Lower Keys marsh rabbit (Sylvilagus palustris hefneri). We observed 5 unique mitochondrial haplotypes across different sampling sites in the Lower Florida Keys, USA. Based on the frequency of these haplotypes at different geographic locations and relationships among haplotypes, we observed 2 distinct clades or groups of sampling sites (western and eastern clades). These 2 groups showed low levels of gene flow. Regardless of their origin, marsh rabbits from the Lower Florida Keys can be separated into 2 genetically distinct management units, which should be considered prior to implementation of translocations as a means of offsetting recent population declines. (JOURNAL OF WILDLIFE MANAGEMENT 73(3):362–367; 2009) DOI: 10.2193/2007-207 KEY WORDS Florida Keys, genetic, marsh rabbit, mitochondrial DNA, population structure, Sylvilagus palustris hefneri. The Lower Keys marsh rabbit (Sylvilagus palustris hefneri)is (Forys and Humphrey 1999b). -

Phylogenetic Study of African Combretaceae R. Br. Based on /.../ A
BALTIC FORESTRY PHYLOGENETIC STUDY OF AFRICAN COMBRETACEAE R. BR. BASED ON /.../ A. O. ONEFELY AND A. STANYS ARTICLES Phylogenetic Study of African Combretaceae R. Br. Based on rbcL Sequence ALFRED OSSAI ONEFELI*,1,2 AND VIDMANTAS STANYS2,3 1Department of Forest Production and Products, Faculty of Renewable Natural Resources, University of Ibadan, 200284 Ibadan, Nigeria. 2Erasmus+ Scholar, Institute of Agricultural and Food Science Vytautas Magnus University, Agricultural Aca- demy, Akademija, LT-53361 Kaunas district, Lithuania. 3Department of Orchard Plant Genetics and Biotechnology, Lithuanian Research Centre for Agriculture and Forestry, Babtai, LT-54333 Kaunas district, Lithuania. *Corresponding author: [email protected], [email protected] Phone number: +37062129627 Onefeli, A. O. and Stanys, A. 2019. Phylogenetic Study of African Combretaceae R. Br. Based on rbcL Se- quence. Baltic Forestry 25(2): 170177. Abstract Combretaceae R. Br. is an angiosperm family of high economic value. However, there is dearth of information on the phylogenetic relationship of the members of this family using ribulose biphosphate carboxylase (rbcL) gene. Previous studies with electrophoretic-based and morphological markers revealed that this family is phylogenetically complex. In the present study, 79 sequences of rbcL were used to study the phylogenetic relationship among the members of Combretaceae of African origin with a view to provide more information required for the utilization and management of this family. Multiple Sequence alignment was executed using the MUSCLE component of Molecular Evolutionary Genetics Version X Analysis (MEGA X). Transition/Transversion ratio, Consistency index, Retention Index and Composite Index were also determined. Phylogenetic trees were constructed using Maximum parsimony (MP) and Neighbor joining methods. -

A Preliminary List of the Vascular Plants and Wildlife at the Village Of
A Floristic Evaluation of the Natural Plant Communities and Grounds Occurring at The Key West Botanical Garden, Stock Island, Monroe County, Florida Steven W. Woodmansee [email protected] January 20, 2006 Submitted by The Institute for Regional Conservation 22601 S.W. 152 Avenue, Miami, Florida 33170 George D. Gann, Executive Director Submitted to CarolAnn Sharkey Key West Botanical Garden 5210 College Road Key West, Florida 33040 and Kate Marks Heritage Preservation 1012 14th Street, NW, Suite 1200 Washington DC 20005 Introduction The Key West Botanical Garden (KWBG) is located at 5210 College Road on Stock Island, Monroe County, Florida. It is a 7.5 acre conservation area, owned by the City of Key West. The KWBG requested that The Institute for Regional Conservation (IRC) conduct a floristic evaluation of its natural areas and grounds and to provide recommendations. Study Design On August 9-10, 2005 an inventory of all vascular plants was conducted at the KWBG. All areas of the KWBG were visited, including the newly acquired property to the south. Special attention was paid toward the remnant natural habitats. A preliminary plant list was established. Plant taxonomy generally follows Wunderlin (1998) and Bailey et al. (1976). Results Five distinct habitats were recorded for the KWBG. Two of which are human altered and are artificial being classified as developed upland and modified wetland. In addition, three natural habitats are found at the KWBG. They are coastal berm (here termed buttonwood hammock), rockland hammock, and tidal swamp habitats. Developed and Modified Habitats Garden and Developed Upland Areas The developed upland portions include the maintained garden areas as well as the cleared parking areas, building edges, and paths. -

Impacts of a Half Century of Sealevel Rise and Development on An
Global Change Biology (2012) 18, 3536–3542, doi: 10.1111/gcb.12024 Impacts of a half century of sea-level rise and development on an endangered mammal JASON A. SCHMIDT*, ROBERT MCCLEERY† ,JENNIFERR.SEAVEY† ,SUSANE.CAMERON DEVITT† andPAIGE M. SCHMIDT‡ *Department of Wildlife and Fisheries Sciences, Texas A&M University, College Station, TX 77843, USA, †Department of Wildlife Ecology and Conservation, University of Florida, 314 Newins-Ziegler Hall, PO Box 110430, Gainesville, FL 32611, USA, ‡Division of Biological Sciences, United States Fish and Wildlife Service, National Wildlife Refuge System, Tulsa, OK 74129, USA Abstract The extraordinary growth of human populations and development in coastal areas over the last half century has eliminated and degraded coastal habitats and threatened the persistence of associated wildlife. Moreover, human-induced sea-level rise (SLR) is projected to further eliminate and alter the same coastal ecosystems, especially low-lying regions. Whereas habitat loss and wildlife population declines from development are well documented, contemporary SLR has not yet been implicated in declines of coastal faunal populations. In addition, the projection of severe synergistic impacts from the combination of development and SLR is well described, yet the scientific litera- ture offers little empirical evidence of the influence of these forces on coastal wildlife. Analysis of aerial photographs from 1959 to 2006 provided evidence of a 64% net loss of the endangered Lower Keys marsh rabbit’s (Sylvilagus palustris hefneri; LKMR) habitat, the majority due to SLR (>48%). Furthermore, there was a strong negative relation- ship between the proportion of development per island and the amount of new habitat formed. -

Guide to Theecological Systemsof Puerto Rico
United States Department of Agriculture Guide to the Forest Service Ecological Systems International Institute of Tropical Forestry of Puerto Rico General Technical Report IITF-GTR-35 June 2009 Gary L. Miller and Ariel E. Lugo The Forest Service of the U.S. Department of Agriculture is dedicated to the principle of multiple use management of the Nation’s forest resources for sustained yields of wood, water, forage, wildlife, and recreation. Through forestry research, cooperation with the States and private forest owners, and management of the National Forests and national grasslands, it strives—as directed by Congress—to provide increasingly greater service to a growing Nation. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable sex, marital status, familial status, parental status, religion, sexual orientation genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD).To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W. Washington, DC 20250-9410 or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer. Authors Gary L. Miller is a professor, University of North Carolina, Environmental Studies, One University Heights, Asheville, NC 28804-3299. -
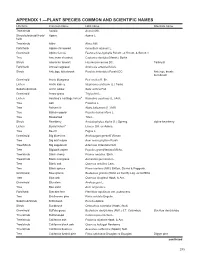
APPENDIX 1.—PLANT SPECIES COMMON and SCIENTIFIC NAMES Life Form Common Name Latin Name Alternate Name Tree/Shrub Acacia Acacia Mill
APPENDIX 1.—PLANT SPECIES COMMON AND SCIENTIFIC NAMES Life form Common name Latin name Alternate name Tree/shrub Acacia Acacia Mill. Shrub/subshrub//Forb/ Agave Agave L. herb Tree/shrub Alder Alnus Mill. Forb/herb Alpine chickweed Cerastium alpinum L. Graminoid Alpine fescue Festuca brachyphylla Schult. ex Schult. & Schult. f. Tree American chestnut Castanea dentata (Marsh.) Borkh. Shrub American tarwort Flourensia cernua DC. Tarbrush Forb/herb Annual ragweed Ambrosia artemisiifolia L. Shrub Antelope bitterbrush Purshia tridentata (Pursh) DC. Antelope brush; buckbrush Graminoid Arctic bluegrass Poa arctica R. Br. Lichen Arctic kidney Nephroma arcticum (L.) Torss. Subshrub/shrub Arctic willow Salix arctica Pall. Graminoid Arrow grass Triglochin L. Lichen Asahina’s cartilage lichend Ramalina asahinae (L.) Ach. Tree Ash Fraxinus L. Tree Balsam fi r Abies balsamea (L.) Mill. Tree Balsam poplar Populus balsamifera L. Tree Basswood Tilia L. Shrub Bearberry Arctostaphylos alpina (L.) Spreng. alpine bearberry Lichen Beard lichena Usnea Dill. ex Adans. Tree Beech Fagus L. Graminoid Big bluestem Andropogon gerardii Vitman Tree Big leaf maple Acer macrophyllum Pursh Tree/Shrub Big sagebrush Artemisia tridentata Nutt. Tree Bigtooth aspen Populus grandidentata Michx. Tree/shrub Black cherry Prunus serotina, Ehrh. Tree/shrub Black mangrove Avicennia germinans L. Tree Black oak Quercus velutina, Lam. Tree Black spruce Picea mariana (Mill.) Britton, Sterns & Poggenb. Graminoid Blue grama Bouteloua gracilis (Willd. ex Kunth) Lag. ex Griffi ths Tree Blue oak Quercus douglasii Hook. & Arn. Graminoid Bluestem Andropogon L. Tree Box elder Acer negundo L. Forb/herb Bracken fern Pteridium aquilinum var. pubescens Tree Bristlecone pine Pinus aristata Engelm. Subshrub/Shrub Brittlebush Encelia Adans. -

Combretaceae: Phylogeny, Biogeography and DNA
COPYRIGHT AND CITATION CONSIDERATIONS FOR THIS THESIS/ DISSERTATION o Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. o NonCommercial — You may not use the material for commercial purposes. o ShareAlike — If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original. How to cite this thesis Surname, Initial(s). (2012) Title of the thesis or dissertation. PhD. (Chemistry)/ M.Sc. (Physics)/ M.A. (Philosophy)/M.Com. (Finance) etc. [Unpublished]: University of Johannesburg. Retrieved from: https://ujdigispace.uj.ac.za (Accessed: Date). Combretaceae: Phylogeny, Biogeography and DNA Barcoding by JEPHRIS GERE THESIS Submitted in fulfilment of the requirements for the degree PHILOSOPHIAE DOCTOR in BOTANY in the Faculty of Science at the University of Johannesburg December 2013 Supervisor: Prof Michelle van der Bank Co-supervisor: Dr Olivier Maurin Declaration I declare that this thesis has been composed by me and the work contained within, unless otherwise stated, is my own. _____________________ J. Gere (December 2013) Table of contents Table of contents i Abstract v Foreword vii Index to figures ix Index to tables xv Acknowledgements xviii List of abbreviations xxi Chapter 1: General introduction and objectives 1.1 General introduction 1 1.2 Vegetative morphology 2 1.2.1 Leaf morphology and anatomy 2 1.2.2. Inflorescence 3 1.2.3 Fruit morphology 4 1.3 DNA barcoding 5 1.4 Cytology 6 1.5 Fossil record 7 1.6 Distribution and habitat 7 1.7 Economic Importance 8 1.8 Taxonomic history 9 1.9 Aims and objectives of the study 11 i Table of contents Chapter 2: Molecular phylogeny of Combretaceae with implications for infrageneric classification within subtribe Terminaliinae. -

Woody and Herbaceous Plants Native to Haiti for Use in Miami-Dade Landscapes1
Woody and Herbaceous Plants Native to Haiti For use in Miami-Dade Landscapes1 Haiti occupies the western one third of the island of Hispaniola with the Dominican Republic the remainder. Of all the islands within the Caribbean basin Hispaniola possesses the most varied flora after that of Cuba. The plants contained in this review have been recorded as native to Haiti, though some may now have been extirpated due in large part to severe deforestation. Less than 1.5% of the country’s original tree-cover remains. Haiti’s future is critically tied to re- forestation; loss of tree cover has been so profound that exotic fast growing trees, rather than native species, are being used to halt soil erosion and lessen the risk of mudslides. For more information concerning Haiti’s ecological plight consult references at the end of this document. For present purposes all of the trees listed below are native to Haiti, which is why non-natives such as mango (the most widely planted tree) and other important trees such as citrus, kassod tree (Senna siamea) and lead tree (Leucanea leucocephala) are not included. The latter two trees are among the fast growing species used for re-forestation. The Smithsonian National Museum of Natural History’s Flora of the West Indies was an invaluable tool in assessing the range of plants native to Haiti. Not surprisingly many of the listed trees and shrubs 1 John McLaughlin Ph.D. U.F./Miami-Dade County Extension Office, Homestead, FL 33030 Page | 1 are found in other parts of the Caribbean with some also native to South Florida.