(12) Patent Application Publication (10) Pub. No.: US 2008/0312247 A1 Gant Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tolerance and Resistance to Organic Nitrates in Human Blood Vessels
\ö-\2- Tolerance and Resistance to Organic Nitrates in Human Blood Vessels Peter Radford Sage MBBS, FRACP Thesis submit.ted for the degree of Doctor of Philosuphy Department of Medicine University of Adelaide and Cardiology Unit The Queen Elizabeth Hospital I Table of Gontents Summary vii Declaration x Acknowledgments xi Abbreviations xil Publications xtil. l.INTRODUCTION l.L Historical Perspective I i.2 Chemical Structure and Available Preparations I 1.3 Cellular/biochemical mechanism of action 2 1.3.1 What is the pharmacologically active moiety? 3 1.3.2 How i.s the active moiety formed? i 4 1.3.3 Which enzyme system(s) is involved in nitrate bioconversi<¡n? 5 1.3.4 What is the role of sulphydryl groups in nitrate action? 9 1.3.5 Cellular mechanism of action after release of the active moiety 11 1.4 Pharmacokinetics t2 1.5 Pharmacological Effects r5 1.5.1 Vascular effects 15 l.5.2Platelet Effects t7 1.5.3 Myocardial effects 18 1.6 Clinical Efhcacy 18 1.6.1 Stable angina pectoris 18 1.6.2 Unstable angina pectoris 2t 1.6.3 Acute myocardial infarction 2l 1.6.4 Congestive Heart Failure 23 ll 1.6.5 Other 24 1.7 Relationship with the endothelium and EDRF 24 1.7.1 EDRF and the endothelium 24 1.7.2 Nitrate-endothelium interactions 2l 1.8 Factors limiting nitrate efficacy' Nitrate tolerance 28 1.8.1 Historical notes 28 1.8.2 Clinical evidence for nitrate tolerance 29 1.8.3 True/cellular nitrate tolerance 31 1.8.3.1 Previous studies 31 | .8.3.2 Postulated mechanisms of true/cellular tolerance JJ 1.8.3.2.1 The "sulphydryl depletion" hypothesis JJ 1.8.3.2.2 Desensitization of guanylate cyclase 35 1 8.i.?..3 Impaired nitrate bioconversion 36 1.8.3.2.4'Ihe "superoxide hypothesis" 38 I.8.3.2.5 Other possible mechanisms 42 1.8.4 Pseudotolerance ; 42 1.8.4. -

Drug Class Review Beta Adrenergic Blockers
Drug Class Review Beta Adrenergic Blockers Final Report Update 4 July 2009 Update 3: September 2007 Update 2: May 2005 Update 1: September 2004 Original Report: September 2003 The literature on this topic is scanned periodically. The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use, or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Mark Helfand, MD, MPH Kim Peterson, MS Vivian Christensen, PhD Tracy Dana, MLS Sujata Thakurta, MPA:HA Drug Effectiveness Review Project Marian McDonagh, PharmD, Principal Investigator Oregon Evidence-based Practice Center Mark Helfand, MD, MPH, Director Oregon Health & Science University Copyright © 2009 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Report Update 4 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION .......................................................................................................................... 6 Purpose and Limitations of Evidence Reports........................................................................................ 8 Scope and Key Questions .................................................................................................................... 10 METHODS................................................................................................................................. -

QT Prolongation Due to Roxithromycin
Postgrad Med J 2000;76:651–654 651 Postgrad Med J: first published as 10.1136/pmj.76.900.651 on 1 October 2000. Downloaded from ADVERSE DRUG REACTION QT prolongation due to roxithromycin A Woywodt, U Grommas, W Buth, W RaZenbeul Roxithromycin and other macrolide antimicro- placement of the apex beat, a prominent third bials are widely used for a broad variety of heart sound, coarse rales over both lung fields infections such as upper respiratory tract infec- and pitting oedema of both ankles. The patient tion and community acquired pneumonia. was taken to an intensive care unit. Acute myo- Prolongation of the QT interval, torsade de cardial infarction was ruled out and frusemide pointes polymorphic ventricular tachycardia, was begun intravenously. An electrocardio- and sudden death are well described but little gram (ECG) on admission showed sinus known adverse reactions common to all rhythm and incomplete left bundle branch macrolides. We report the case of a 72 year old block; QT intervals were normal (QT interval patient with congestive heart failure caused by 380 ms, corrected QT interval according to University of ischaemic heart disease who developed severe Bazett’s formula [QTc] 390 ms). Roxithromy- Hannover Medical prolongation of the QT interval after three days cin (Roussel UCLAF, Romainville, France) School, 30623 of treatment with roxithromycin. 150 mg twice a day was initiated for suspected Hannover, Germany: pneumonia. On the third hospital day, he was Department of Nephrology Case report transferred to a general medical ward. A Woywodt A 72 year old man presented with severe On admission there, the patient was gener- W Buth congestive heart failure. -

Inhibitory Effect of Eslicarbazepine Acetate and S-Licarbazepine on 2 Nav1.5 Channels
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.24.059188; this version posted August 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Inhibitory effect of eslicarbazepine acetate and S-licarbazepine on 2 Nav1.5 channels 3 Theresa K. Leslie1, Lotte Brückner 1, Sangeeta Chawla1,2, William J. Brackenbury1,2* 4 1Department of Biology, University of York, Heslington, York, YO10 5DD, UK 5 2York Biomedical Research Institute, University of York, Heslington, York, YO10 5DD, UK 6 * Correspondence: Dr William J. Brackenbury, Department of Biology and York Biomedical 7 Research Institute, University of York, Wentworth Way, Heslington, York YO10 5DD, UK. Email: 8 [email protected]. Tel: +44 1904 328284. 9 Keywords: Anticonvulsant, cancer, epilepsy, eslicarbazepine acetate, Nav1.5, S-licarbazepine, 10 voltage-gated Na+ channel. 11 Abstract 12 Eslicarbazepine acetate (ESL) is a dibenzazepine anticonvulsant approved as adjunctive treatment for 13 partial-onset epileptic seizures. Following first pass hydrolysis of ESL, S-licarbazepine (S-Lic) 14 represents around 95 % of circulating active metabolites. S-Lic is the main enantiomer responsible 15 for anticonvulsant activity and this is proposed to be through the blockade of voltage-gated Na+ 16 channels (VGSCs). ESL and S-Lic both have a voltage-dependent inhibitory effect on the Na+ current 17 in N1E-115 neuroblastoma cells expressing neuronal VGSC subtypes including Nav1.1, Nav1.2, 18 Nav1.3, Nav1.6 and Nav1.7. -

Alphabetical Listing by Therapeutic Category GOODFELLOW AFB ROSS CLINIC FORMULARY
GOODFELLOW AFB ROSS CLINIC FORMULARY Alphabetical Listing by Therapeutic Category This document is current as of October 8, 2020. The availability of formulary items is subject to change. ACARBOSE Acarbose ALPRAZolam Outpatient Dosage Forms Outpatient Formulary Brands Available Xanax Tablet, Oral: Outpatient Dosage Forms Generic: 25 mg, 50 mg, 100 mg Tablet, Oral: Xanax: 0.5 mg, 1 mg [scored] Acetaminophen Generic: 0.5 mg, 1 mg Outpatient Formulary Brands Available Mapap Children's [OTC]; Mapap [OTC] Aluminum Chloride Hexahydrate Outpatient Dosage Forms Outpatient Formulary Brands Available Drysol Suspension, Oral: Outpatient Dosage Forms Mapap Children's: 160 mg/5 mL (118 mL) [ethanol free; contains propylene Solution, External: glycol, sodium benzoate; cherry flavor] Drysol: 20% (60 mL) Tablet, Oral: Mapap: 325 mg Aluminum Hydroxide, Magnesium Hydroxide, and Simethicone Generic: 325 mg Outpatient Dosage Forms Liquid, Oral: Acetaminophen and Codeine Generic: Aluminum hydroxide 200 mg, magnesium hydroxide 200 mg, and Outpatient Dosage Forms simethicone 20 mg per 5 mL (360 mL) Solution, Oral [C-V]: Generic: Acetaminophen 120 mg and codeine phosphate 12 mg per 5 mL Amantadine (118 mL) [BCF] Outpatient Dosage Forms Tablet, Oral [C-III]: Capsule, Oral, as hydrochloride: Generic: Acetaminophen 300 mg and codeine phosphate 30 mg [BCF] Generic: 100 mg [BCF] AcetaZOLAMIDE Amiodarone Outpatient Dosage Forms Outpatient Dosage Forms Tablet, Oral: Tablet, Oral, as hydrochloride: Generic: 250 mg Generic: 200 mg [BCF] Acetic Acid, Propylene Glycol Diacetate, -

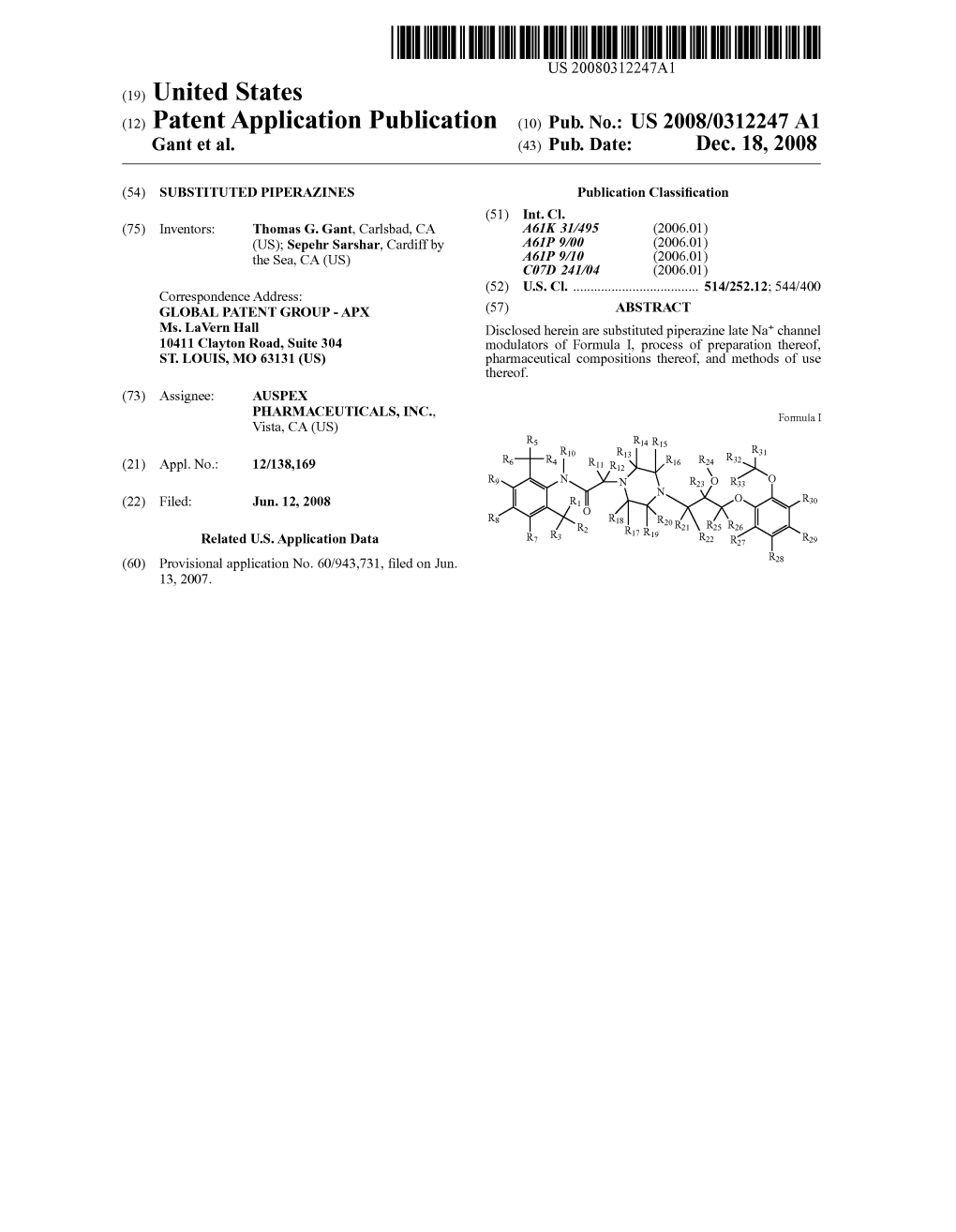
(12) United States Patent (10) Patent No.: US 6,641,839 B1 Geoghegan Et Al
USOO6641839B1 (12) United States Patent (10) Patent No.: US 6,641,839 B1 Geoghegan et al. (45) Date of Patent: Nov. 4, 2003 (54) PHARMACEUTICAL FORMULATIONS FOR JP 59 84.820 5/1984 PREVENTING DRUG TOLERANCE JP 62126127 * 6/1987 (75) Inventors: Edward James Geoghegan, Athlone OTHER PUBLICATIONS (IE); Seamus Mulligan, Athlone (IE); “Isosorbide-5-Nitrate Sustained-release pellets-an Mary Margaret Foynes, Athlone (IE) example of computer Supported drug development', Zerbe et al., Pharmacuetical Resarch 1985, No. 1, Jan., pp. 30–36. (73) Assignee: Athpharma Limited, Roscommon (IE) “Glyceryl trinitrate (nitroglycerine) and the Organic nitrates choosing the method of administration”, J. Abrahms, Drugs (*) Notice: Subject to any disclaimer, the term of this 34; 391-403 (1987). patent is extended or adjusted under 35 “Dose Dependence of Tolerance during treatment with U.S.C. 154(b) by 407 days. mono-nitrates', M. Tauchert et al., Z. Cardiol. 72, Suppl. 3, 218–228 (1983). (21) Appl. No.: 08/797,318 “Tolerance Development during isosorbide dinitrate treat ment: Can it be circumvented?”, W. Rudolf et al., Z. Cardiol. (22) Filed: Feb. 7, 1997 72, Supple. 3, 195-198 (1983). Related U.S. Application Data “Anti-ischemic effects of 80mg tablet of isosorbide dinitrate in Sustained-release form before and after two weeks treat (63) Continuation of application No. 08/419,520, filed on Apr. ment with 80mg once daily or twice daily', S.Silver et al., 10, 1995, which is a continuation of application No. 08/320, Z.Cardiol. 72, Suppl. 3, 211-217 (1983). 599, filed on Oct. 11, 1994, which is a continuation of application No. -

Cardiovascular Drugs and Therapies NITRATES COMPARISON CHART
Cardiovascular Drugs and Therapies NITRATES COMPARISON CHART Isosorbide Generic Nitroglycerin Nitroglycerin Nitroglycerin Nitroglycerin Dinitrate Isosorbide Isosorbide Name Intravenous Patch Ointment Sublingual Sublingual Dinitrate 5-Mononitrate Trade Name TRIDIL, NITRODUR, NITROL NITROLINGUAL generics generics (for IMDUR, generics TRANSDERM- Pumpspray, immediate generics NITRO, RHO-NITRO release) MINITRAN, Pumpspray, SR: no longer TRINIPATCH NITROLINGUAL available Metered dose spray NITROSTAT sublingual tablet Dosage 100 mg/250 mL 0.2 mg/h 30 g/30 inches SL spray: SL tablet: 5mg Immediate SR tablet: Forms premixed bottle 0.4 mg/h ointment 0.4 mg/ dose release 60 mg SR - UHN 0.6 mg/h SL tablet: tablet: Note: 0.84 mL 0.8 mg/h 0.3 mg, 0.6 mg 10 mg *Non- alcohol per 100 mL 30 mg formulary at solution UHN 100 mcg/mL 200 mcg/mL 400 mcg/mL 10 mg/10 mL vial - UHN 50 mg/10 mL vial - UHN CARDIOVASCULAR PHARMACOTHERAPY HANDBOOK All contents copyright © University Health Network. All rights reserved Cardiovascular Drugs and Therapies NITRATES COMPARISON CHART Isosorbide Generic Nitroglycerin Nitroglycerin Nitroglycerin Nitroglycerin Dinitrate Isosorbide Isosorbide Name Intravenous Patch Ointment Sublingual Sublingual Dinitrate 5-Mononitrate Dosing Starting and 0.2 to 0.8 ½ inch to 1 inch SL spray: SL tablet: Immediate 60-240 mg SR Usual dose target doses mg/h once tid-qid; remove 0.4 mg prn; 5-10 mg q2-4h release: once daily range are determined daily. for 8-10 hours dose may be for prophylaxis 10-45 mg tid by clinical per 24-hour repeated after of acute angina on qid situation and 12-14 hour period; 5 minutes for schedule the number and patch-free response to interval e.g., ON 0600, total of 3 doses (e.g. -

Summary of Product Characteristics
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT *IPERTEN/ARTEDIL/MANYPER 10mg tablets IPERTEN/ARTEDIL/MANYPER 20mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION IPERTEN/ARTEDIL/MANYPER 10 mg tablets Each tablet contains: Manidipine hydrochloride 10mg Excipient with known effect: 119,61 mg lactose monohydrate/tablet IPERTEN/ARTEDIL/MANYPER 20 mg tablets Each tablet contains: Manidipine hydrochloride 20mg Excipient with known effect: 131,80 mg lactose monohydrate/tablet For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet IPERTEN/ARTEDIL/MANYPER 10mg: pale yellow, round, scored tablet; IPERTEN/ARTEDIL/MANYPER 20mg: yellow-orange, oblong, scored tablet. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Mild to moderate essential hypertension 4.2 Posology and method of administration The recommended starting dose is 10 mg once a day. Should the antihypertensive effect be still insufficient after 2-4 weeks of treatment, it is advisable to increase the dosage to the usual maintenance dose of 20 mg once a day. Elderly In view of the slowing down of metabolism in the elderly, the recommended dose is 10mg once daily. This dosage is sufficient in most elderly patients; the risk/benefit of any dose increase should be considered with caution on an individual basis. Renal impairment In patients with mild to moderate renal dysfunction care should be taken when increasing the dosage from 10 to 20mg once a day. Hepatic impairment Due to the extensive hepatic metabolisation of manidipine, patients with mild hepatic dysfunction should not exceed 10mg once a day (see also Section 4.3 Contraindications). Tablet must be swallowed in the morning after breakfast, without chewing it, with a few liquid. -

Amyl Nitrite Or 'Jungle Juice'
Young People and Other Drugs Amyl Nitrite or ‘Jungle Juice’ Amyl nitrite is an inhalant that belongs to a class As with any drug, the use of nitrites is not risk-free. of chemicals called alkyl nitrites. This group of Some of the harms associated with its use include: drugs can be called ‘poppers’. They are often injuries related to inhaling the vapour referred to by their brand name, with ‘Jungle (e.g., rashes, burns) Juice’ probably being the most well-known of these. allergic reactions accidents and falls Inhaling amyl nitrite relaxes the body and gives vision problems (isopropyl nitrite) a ‘rush’ that lasts for one to two minutes. It is commonly used to enhance sexual pleasure and in rare cases, blood disorders induce a feeling of euphoria and well-being. MOST IMPORTANTLY, AMYL NITRITE OR JUNGLE JUICE MUST NEVER BE DRUNK. Drinking amyl can result in death due to it interfering with the ability of the blood to transport oxygen. What is amyl nitrite? Over the years, to bypass legal restrictions, nitrites have been sold as such things as liquid incense or Amyl nitrite is an inhalant that belongs to a class of room odoriser. Jungle Juice, which can be sold as chemicals called alkyl nitrites. Amyl nitrite is a highly a leather cleaner, is a common product name of flammable liquid that is clear or yellowish in colour. amyl nitrite. It has a unique smell that is sometimes described as ‘dirty socks’. It is highly volatile and when exposed to the air evaporates almost immediately at How is Jungle Juice used? room temperature. -

Primary-Explosives
Improvised Primary Explosives © 1998 Dirk Goldmann No part of the added copyrighted parts (except brief passages that a reviewer may quote in a review) may be reproduced in any form unless the reproduced material includes the following two sentences: The copyrighted material may be reproduced without obtaining permission from anyone, provided: (1) all copyrighted material is reproduced full-scale. WARNING! Explosives are danegerous. In most countries it's forbidden to make them. Use your mind. You as an explosives expert should know that. 2 CONTENTS Primary Explosives ACETONE PEROXIDE 4 DDNP/DINOL 6 DOUBLE SALTS 7 HMTD 9 LEAD AZIDE 11 LEAD PICRATE 13 MEKAP 14 MERCURY FULMINATE 15 "MILK BOOSTER" 16 NITROMANNITE 17 SODIUM AZIDE 19 TACC 20 Exotic and Friction Primers LEAD NITROANILATE 22 NITROGEN SULFIDE 24 NITROSOGUANIDINE 25 TETRACENE 27 CHLORATE-FRICTION PRIMERS 28 CHLORATE-TRIMERCURY-ACETYLIDE 29 TRIHYDRAZINE-ZINC (II) NITRATE 29 Fun and Touch Explosives CHLORATE IMPACT EXPLOSIVES 31 COPPER ACETYLIDE 32 DIAMMINESILVER II CHLORATE 33 FULMINATING COPPER 33 FULMINATING GOLD 34 FULMINATING MERCURY 35 FULMINATING SILVER 35 NITROGEN TRICHLORIDE 36 NITROGEN TRIIODIDE 37 SILVER ACETYLIDE 38 SILVER FULMINATE 38 "YELLOW POWDER" 40 Latest Additions 41 End 3 PRIMARY EXPLOSIVES ACETONE PEROXIDE Synonyms: tricycloacetone peroxide, acetontriperoxide, peroxyacetone, acetone hydrogen explosive FORMULA: C9H18O6 VoD: 3570 m/s @ 0.92 g/cc. 5300 m/s @ 1.18 g/cc. EQUIVALENCE: 1 gram = No. 8 cap .75 g. = No. 6 cap SENSITIVITY: Very sensitive to friction, flame and shock; burns violently and can detonate even in small amounts when dry. DRAWBACKS: in 10 days at room temp. 50 % sublimates; it is best made immediately before use. -

Irreversible Activation and Stabilization of Soluble Guanylate Cyclase by The
Molecular Pharmacology Fast Forward. Published on November 14, 2017 as DOI: 10.1124/mol.117.109918 This article has not been copyedited and formatted. The final version may differ from this version. MOL #109918 Irreversible activation and stabilization of soluble guanylate cyclase by the protoporphyrin IX mimetic cinaciguat Alexander Kollau, Marissa Opelt, Gerald Wölkart, Antonius C.F. Gorren, Michael Russwurm, Doris Koesling, Bernd Mayer and Astrid Schrammel Downloaded from Department of Pharmacology and Toxicology, University of Graz, Austria molpharm.aspetjournals.org (A.K., M.O., G.W., A.C.F.G., B.M., A.S.) Department of Pharmacology and Toxicology, Ruhr University Bochum, Bochum, Germany (M.R., D.K.) at ASPET Journals on September 29, 2021 1 Molecular Pharmacology Fast Forward. Published on November 14, 2017 as DOI: 10.1124/mol.117.109918 This article has not been copyedited and formatted. The final version may differ from this version. MOL #109918 Running Title: Irreversible activation of sGC by cinaciguat Address correspondence to: Dr. Astrid Schrammel Department of Pharmacology and Toxicology Karl-Franzens-Universität Graz Humboldtstrasse 46, A-8010 Graz, Austria Downloaded from Tel.: +43-316-380-5559 Fax: +43-316-380-9890 e-mail: [email protected] molpharm.aspetjournals.org Number of text pages: 24 Number of tables: – at ASPET Journals on September 29, 2021 Number of figures: 3 Number of references: 27 Number of words in Abstract: 236 Introduction: 436 Discussion: 904 1Abbreviations: DEA/NO, 2,2-diethyl-1-nitroso-oxyhydrazine; DTT, dithiothreitol; IBMX, 3-isobutyl-1-methylxanthin; NO, nitric oxide; ODQ, 1H-[1,2,4]oxadiazolo-[4,3- a]quinoxalin-1-one; PDE, phosphodiesterase; sGC, soluble guanylate cyclase; 2 Molecular Pharmacology Fast Forward. -

The Repurposing Drugs in Oncology Database
ReDO_DB: the repurposing drugs in oncology database Pan Pantziarka1,2, Ciska Verbaanderd1,3, Vidula Sukhatme4, Rica Capistrano I1, Sergio Crispino1, Bishal Gyawali1,5, Ilse Rooman1,6, An MT Van Nuffel1, Lydie Meheus1, Vikas P Sukhatme4,7 and Gauthier Bouche1 1The Anticancer Fund, Brussels, 1853 Strombeek-Bever, Belgium 2The George Pantziarka TP53 Trust, London, UK 3Clinical Pharmacology and Pharmacotherapy, Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium 4GlobalCures Inc., Newton, MA 02459 USA 5Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115 USA 6Oncology Research Centre, Vrije Universiteit Brussel, Brussels, Belgium 7Emory University School of Medicine, Atlanta, GA 30322 USA Correspondence to: Pan Pantziarka. Email: [email protected] Abstract Repurposing is a drug development strategy that seeks to use existing medications for new indications. In oncology, there is an increased level of activity looking at the use of non-cancer drugs as possible cancer treatments. The Repurposing Drugs in Oncology (ReDO) project has used a literature-based approach to identify licensed non-cancer drugs with published evidence of anticancer activity. Data from 268 drugs have been included in a database (ReDO_DB) developed by the ReDO project. Summary results are outlined and an assessment Research of clinical trial activity also described. The database has been made available as an online open-access resource (http://www.redo-project. org/db/). Keywords: drug repurposing, repositioning, ReDO project, cancer drugs, online database Published: 06/12/2018 Received: 27/09/2018 ecancer 2018, 12:886 https://doi.org/10.3332/ecancer.2018.886 Copyright: © the authors; licensee ecancermedicalscience.