International Standard Iso 23443:2020(E)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BSI Standards Publication
BS ISO 23443:2020 BSI Standards Publication Infant formula and adult nutritionals — Determination of β-carotene, lycopene and lutein by reversed-phase ultra-high performance liquid chromatography (RP-UHPLC) BS ISO 23443:2020 BRITISH STANDARD INTERNATIONAL ISO STANDARD 23443 National foreword This British Standard is the UK implementation of ISO 23443:2020. First edition 2020-07 The UK participation in its preparation was entrusted to Technical Committee AW/34, Food Technical Committee Chairmen. A list of organizations represented on this committee can be obtained on request to its committee manager. This publication does not purport to include all the necessary provisions of a contract. Users are responsible for its correct application. © The British Standards Institution 2020 Published by BSI Standards Limited 2020 Infant formula and adult ISBN 978 0 539 05212 1 nutritionals — Determination of ICS 67.050 β-carotene, lycopene and lutein Compliance with a British Standard cannot confer immunity from by reversed-phase ultra-high legal obligations. performance liquid chromatography This British Standard was published under the authority of the Standards Policy and Strategy Committee on 31 July 2020. (RP-UHPLC) Formules infantiles et produits nutritionnels pour adultes — Amendments/corrigenda issued since publication Détermination du bêta-carotène, du lycopène et de la lutéine par Date Text affected chromatographie liquide ultra haute performance à phase inversée Reference number ISO 23443:2020(E) © ISO 2020 BS ISO 23443:2020 INTERNATIONAL -

Natural Colour Book
THE COLOUR BOOK Sensient Food Colors Europe INDEX NATURAL COLOURS AND COLOURING FOODS INDEX 46 Lycopene 4 We Brighten Your World 47 Antho Blends – Pink Shade 6 Naturally Different 48 Red Cabbage 8 The Colour of Innovation 49 Beetroot – with reduced bluish tone 10 Natural Colours, Colouring Foods 50 Beetroot 11 Cardea™, Pure-S™ 51 Black Carrot 12 YELLOW 52 Grape 14 Colourful Impulses 53 Enocianin 15 Carthamus 54 Red Blends 16 Curcumin 56 VIOLET & BLUE 17 Riboflavin 59 Violet Blends 18 Lutein 61 Spirulina 19 Carrot 62 GREEN 20 Natural Carotene 65 Green Blends 22 Beta-Carotene 66 Copper-Chlorophyllin 24 Annatto 67 Copper-Chlorophyll 25 Yellow/ Orange Blends 68 Chlorophyll/-in 26 ORANGE 69 Spinach 29 Natural Carotene 70 BROWN 30 Paprika Extract 73 Burnt Sugar 32 Carrot 74 Apple 33 Apocarotenal 75 Caramel 34 Carminic Acid 76 BLACK & WHITE 35 Beta-Carotene 79 Vegetable Carbon 36 RED 80 Titanium Dioxide 39 Antho Blends – Strawberry Shade 81 Natural White 40 Aronia 41 Elderberry 83 Regulatory Information 42 Black Carrot 84 Disclaimer 43 Hibiscus 85 Contact Address 44 Carmine 3 INDEX NATURAL COLOURS AND COLOURING FOODS WE BRIGHTEN YOUR WORLD Sensient is as colourful as the world around us. Whatever you are looking for, across the whole spectrum of colour use, we can deliver colouring solutions to best meet your needs in your market. Operating in the global market place for over 100 years Sensient both promises and delivers proven international experience, expertise and capabilities in product development, supply chain management, manufacture, quality management and application excellence of innovative colours for food and beverages. -

Assessing the Fatty Acid, Carotenoid, and Tocopherol Compositions of Seeds from Apple Cultivars (Malus Domestica Borkh.) Grown in Norway
foods Article Assessing the Fatty Acid, Carotenoid, and Tocopherol Compositions of Seeds from Apple Cultivars (Malus domestica Borkh.) Grown in Norway Milica Fotiri´cAkši´c 1, Kristina Lazarevi´c 2, Sandra Šegan 3 , Maja Nati´c 4 , Tomislav Tosti 4 , Ivanka Ciri´c´ 5 and Mekjell Meland 6,* 1 Faculty of Agriculture, University of Belgrade, 11080 Belgrade, Serbia; [email protected] 2 Centre for Food Analysis, 11080 Belgrade, Serbia; [email protected] 3 Institute of Chemistry, Technology, and Metallurgy, University of Belgrade, 11000 Belgrade, Serbia; [email protected] 4 Faculty of Chemistry, University of Belgrade, 11000 Belgrade, Serbia; [email protected] (M.N.); [email protected] (T.T.) 5 Innovation Centre of Faculty of Chemistry Ltd., 11000 Belgrade, Serbia; [email protected] 6 Norwegian Institute of Bioeconomy Research, NIBIO Ullensvang, Ullensvangvegen 1003, N-5781 Lofthus, Norway * Correspondence: [email protected] Abstract: Apple production generates large amounts of apple pomace including seeds, leading to high transportation costs, public health hazards and undesirable odor. A new reuse strategy of this kind of waste could solve environmental issues and/or create unconventional sources of health Citation: Akši´c,M.F.; Lazarevi´c,K.; beneficial products. In total, seeds from 75 apple cultivars grown in Norway (both domestic and Šegan, S.; Nati´c,M.; Tosti, T.; Ciri´c,I.;´ international) have been analyzed for the first time for oil content and fatty acid profile together with Meland, M. Assessing the Fatty Acid, tocopherols and carotenoids quantification in defatted seeds. Seeds from cultivar Håkonseple had Carotenoid, and Tocopherol the highest oil content (22.10%), with linoleic, oleic acid, and palmitic acid as the most abundant Compositions of Seeds from Apple β α Cultivars (Malus domestica Borkh.) fatty acids. -

Apocarotenoids: a Brief Review
International Journal of Research and Review Vol.7; Issue: 12; December 2020 Website: www.ijrrjournal.com Review Article E-ISSN: 2349-9788; P-ISSN: 2454-2237 Apocarotenoids: A Brief Review Seba M C, Anatt Treesa Mathew, Sheeja Rekha, Prasobh G R Department of Pharmaceutical Chemistry, Sree Krishna College of Pharmacy and Research Centre, Parassala, Kerala Corresponding Author: Seba M C ABSTRACT The development of apocarotenoids is started by oxidative cleavage reactions Carotenoids are broad group of natural that can be catalyzed by enzymes, generally pigments, extending from red to orange, to CCDs, or take place via exposure of yellow colours. Produced by plants and certain carotenoids to ROS. β-Cyclocitral is an microorganisms (e.g.: fungi, bacteria and example for a carotenoid cleavage product microalgae), carotenoids have significant physiological function (e.g.: light harvesting). that can be produced following both Apocarotenoids are organic compounds, which scenarios. The formation of this β-carotene– is cleavage products of C40 isoprenoid derived volatile in photosynthetic tissues is 1 pigments, named carotenoids, catalyzed by initiated by a singlet oxygen O2 attack, enzyme carotenoid oxygenase, produced which takes place in the photosystem II, absolutely by plants and microorganisms. particularly in the presence of high light Apocarotenoids show vital roles in several stress, while in citrus fruits, it is catalyzed biological activities (e.g., plant hormones). by a CCD (CCD4b) that cleaves β-carotene, Various carotenoids and apocarotenoids have β-cryptoxanthin, and zeaxanthin. Although great economic significance in feed, cosmetics, some CCDs display a quite relaxed substrate food supplements, pharmaceutical industries and and site (double bond) specificity , cleavage also possess high commercial values Key Words: Carotenoids, Apocarotenoids, reactions catalyzed by these enzymes Carotenoid oxygenase generally take place at definite double bond(s) in defined carotenoid/apocarotenoid INTRODUCTION substrate(s). -

(12) United States Patent (10) Patent No.: US 6,426,078 B1 Bauer Et Al
USOO6426078B1 (12) United States Patent (10) Patent No.: US 6,426,078 B1 Bauer et al. (45) Date of Patent: Jul. 30, 2002 (54) OIL IN WATER MICROEMULSION 5,118,511 A 6/1992 Horn et al. ................. 424/502 5,213,799 A * 5/1993 Goring et al. .............. 424/401 (75) Inventors: Kurt Bauer, Freiburg-Tiengen; FOREIGN PATENT DOCUMENTS Clarissa Neuber, Villingen-Schwenningen; Axel Schmid, GB 2 222 770 3/1990 Gottmadingen-Bietingen; Karl Manfred GB 2 297 759 8/1996 Völker, Freiburg, all of (DE) OTHER PUBLICATIONS (73) Assignee: Roche Vitamins Inc., Parsippany, NJ English language astract of JP 8120187. (US) Derwent Abstract No. AN 86-172070. Patent Abstract of Japan No. JP 59051214. (*) Notice: Subject to any disclaimer, the term of this Patent Abstract of Japan No. JP 07204487. patent is extended or adjusted under 35 Derwent Abstract No. AN 87-040923. U.S.C. 154(b) by 0 days. Patent Abstract of Japan No. JP 09157159. Patent Abstract of Japan No. JP 60137260. (21) Appl. No.: 09/030,714 * cited by examiner (22) Filed: Feb. 26, 1998 Primary Examiner. Theodore J. Criares (30) Foreign Application Priority Data ASSistant Examiner Jennifer Kim Mar. 17, 1997 (CH) .............................................. O628/97 (74) Attorney, Agent, or Firm-Byran Cave LLP (51) Int. Cl. .......................... A61K 6/00; A61K 47/00; (57) ABSTRACT A61K 31/74 The invention is concerned with a microemulsion of the (52) U.S. Cl. .................... 424/401; 424/439; 424/78.03; oil-in water type, containing at least one polyglycerol ester 424/78.04; 514/844 as the emulsifier and at least one lipophilic Substance as the (58) Field of Search ................................ -

Anti-Obesity Effect of Carotenoids: Direct Impact on Adipose Tissue
nutrients Review Anti-Obesity Effect of Carotenoids: Direct Impact on Adipose Tissue and Adipose Tissue-Driven Indirect Effects Lourdes Mounien 1, Franck Tourniaire 1,2 and Jean-Francois Landrier 1,2,* 1 Aix Marseille Univ, INSERM, INRA, C2VN, 13385 Marseille, France 2 CriBioM, criblage biologique Marseille, faculté de Médecine de la Timone, 13256 Marseille, France * Correspondence: [email protected]; Tel.: +33-491-324-275 Received: 29 May 2019; Accepted: 7 July 2019; Published: 11 July 2019 Abstract: This review summarizes current knowledge on the biological relevance of carotenoids and some of their metabolites in obesity management. The relationship between carotenoids and obesity is considered in clinical studies and in preclinical studies. Adipose tissue is a key organ in obesity etiology and the main storage site for carotenoids. We thus first describe carotenoid metabolism in adipocyte and adipose tissue and the effects of carotenoids on biological processes in adipose tissue that may be linked to obesity management in in vitro and preclinical studies. It is also now well established that the brain is strongly involved in obesity processes. A section is accordingly devoted to the potential effect of carotenoids on obesity via their direct and/or adipose tissue-driven indirect biological effects on the brain. Keywords: adipocytes; adipose tissue; brain; carotenoids; obesity 1. Obesity, Comorbidities, Adipose Tissue and Brain Dysfunctions The World Health Organization (WHO) defines obesity and being overweight as abnormal or excessive fat accumulation that presents a risk to health [1]. The risk is mainly related to comorbidities strongly linked to obesity such as metabolic inflammation, insulin resistance, liver steatosis, hypertension, dyslipidemia, certain types of cancer, depression, etc. -

Carotene Interference with UVA-Induced Gene Expression by Multiple Pathways*
Pure Appl. Chem., Vol. 78, No. 8, pp. 1539–1550, 2006. doi:10.1351/pac200678081539 © 2006 IUPAC -Carotene interference with UVA-induced gene expression by multiple pathways* Karin Wertz, Nicole Seifert, Petra Buchwald Hunziker, Georges Riss, Adrian Wyss, Willi Hunziker†, and Regina Goralczyk‡ Department of Human Nutrition and Health, DSM Nutritional Products, Kaiseraugst, Switzerland 1 Abstract: UVA exposure causes skin photoaging by singlet oxygen ( O2)-mediated induction of matrix metalloproteases (MMPs). We assessed whether β-carotene, a carotenoid known as 1 O2 quencher and retinoic acid (RA) precursor, interferes with UVA-induced gene regulation and prevents UVA-induced gene regulation in HaCaT human keratinocytes. HaCaT cells ac- cumulated β-carotene in a time- and dose-dependent manner. UVA irradiation massively re- duced the cellular β-carotene contents. β-Carotene suppressed UVA induction of MMP-1, MMP-3, and MMP-10—three major MMPs involved in photoaging. HaCaT cells produced weak retinoid activity from β-carotene, as demonstrated by mild up-regulation of retinoid re- ceptor RARβ and activation of an RARE-dependent reporter gene. Of the 568 UVA-regu- lated genes, β-carotene reduced the UVA effect for 143, enhanced it for 180, and did not in- teract with UVA for 245 genes. The pathways regulated β-carotene in interaction with UVA were characterized by genes involved in growth factor signaling, stress response, apoptosis, cell cycle, extracellular matrix (ECM) degradation, tanning, and inflammation. In conclusion, β-carotene at physiological concentrations interacted with UVA effects 1 by multiple mechanisms that included, but were not restricted to, O2 quenching. With our results, we provide a mechanistic basis for the long-known and clinically established photo- protective effects of β-carotene in human skin. -

Degradation of -Carotene During Fruit and Vegetable Processing Or
Degradation of β-carotene during fruit and vegetable processing or storage: reaction mechanisms and kinetic aspects: a review Caroline Pénicaud, Nawel Achir, Claudie Dhuique-Mayer, Manuel Dornier, Philippe Bohuon To cite this version: Caroline Pénicaud, Nawel Achir, Claudie Dhuique-Mayer, Manuel Dornier, Philippe Bohuon. Degra- dation of β-carotene during fruit and vegetable processing or storage: reaction mechanisms and kinetic aspects: a review. Fruits, EDP Sciences/CIRAD, 2011, 66 (6), pp.417-440. 10.1051/fruits/2011058. hal-01506490 HAL Id: hal-01506490 https://hal.archives-ouvertes.fr/hal-01506490 Submitted on 14 Jun 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Review Degradation of β-carotene during fruit and vegetable processing or storage: reaction mechanisms and kinetic aspects: a review Caroline PÉNICAUD, Nawel ACHIR*, Claudie DHUIQUE-MAYER, Manuel DORNIER, Philippe BOHUON Cirad-Persyst, UMR 95 Degradation of β-carotene during fruit and vegetable processing or storage: reaction Qualisud, TA B-95 / 16, mechanisms and kinetic aspects: a review. 73 rue Jean-François Breton, Abstract — Introduction. Food processing significantly lowers the quality of fruits and vegetables, which F-34398 Montpellier, is a major concern for the food industry. -
Beta Carotene
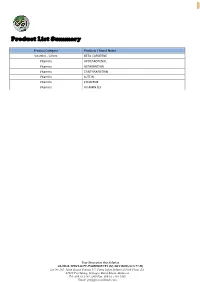
2 Product List Summary Product Category Products \ Brand Name Vitamins , Colors BETA-CAROTENE Vitamins APOCAROTENAL Vitamins ASTAXANTHIN Vitamins CANTHAXANTHIN Vitamins LUTEIN Vitamins LYCOPENE Vitamins VITAMIN D3 Your Innovation Our Solution GLOBAL SPECIALTY INGREDIENTS (M) SDN BHD (832177-M) Lot No 202, Jalan Sungai Pinang 5/7, Pulau Indah Industrial Park Phase 2A, 42920 Port Klang, Selangor Darul Ehsan, Malaysia Tel: 006 03 3101 3500 Fax: 006 03 3101 4500 Email: [email protected] 3 Product Name: BETA CAROTENE. Product Statement: β-Carotene – BETA CAROTENE is a strongly-colored red-orange, fat-soluble compound belonging to the Carotenoid group. It is a precursor of Vitamin A that occurs naturally in plants and fruits. It has potent antioxidant characteristics that can protect cells from the damaging effects of free radicals. Various forms of Beta-Carotene have been created based on customer’s requirement and specifications. Application: Beta-Carotene Formulations Description Application Characteristics Beta-Carotene 30% (corn oil) Soft-gelatine capsules Yellow to orange Beta-Carotene 30% Sun (sunflower oil) Suspension Fortification/ coloration of colour. Beta-Carotene 30% Soy (soybean oil) oil-based foods. Fortification/ coloration of Heat Stable. Beta-Carotene 22% LF (corn oil) Suspension oil-based foods such as pop Yellow to orange corns and frying oil. colour. Coloration of water based Beta-Carotene 1% SD Spray-dried Yellow to yellow- foods, beverages and instant Beta-Carotene 5% SD powder orange colour. products. Fortification/ coloration of Beta-Carotene 10% DG (modified starch) Yellow-orange to Beads water based food and Beta-Carotene 10% DG/F (fish gelatine) orange colour. beverages. -

A Guide to Carotenoid Analysis in Foods
A GUIDE TO CAROTENOIDA GUIDE TO ANAL This publication is made possible by support from Opportunities for Micronutri- ent Interventions (OMNI) Research, a project of the Office of Health and Nutrition, Bureau of Global Programs, Field Support and Re- search, the U.S. Agency for International Development (USAID) under cooperative A GUIDE TO agreement HRN-5122-A-00-3046-00 with the International Life Sciences Institute (ILSI) Research Foundation. CAROTENOID The information presented herein does YSIS IN FOODS not necessarily reflect the scientific recom- mendations or views of OMNI Research, ANALYSIS IN USAID, or ILSI. Additional copies of this and other OMNI Research publications are available FOODS free of charge to developing countries and for US$3.50 to developed countries. Copies can be ordered from OMNI Research at: RODRIGUEZ-AMAYA OMNI Research ILSI Human Nutrition Institute One Thomas Circle, NW Washington, DC 20005-5802, USA Email: [email protected] Internet: hni.ilsi.org/publications Delia B. Rodriguez-Amaya, Ph.D. A GUIDE TO CAROTENOID ANALYSIS IN FOODS Delia B. Rodriguez-Amaya, Ph.D. Departamento de Ciência de Alimentos Faculdade de Engenharia de Alimentos Universidade Estadual de Campinas C.P. 6121, 13083-970 Campinas, SP., Brasil The use of trade names and commercial sources in this document is for purposes of identification only, and does not imply endorse- ment by ILSI. In addition, the views expressed herein are those of the individual authors and/or their organizations, and do not necessarily reflect those of ILSI. Other publications from OMNI Research are available through the ILSI Human Nutrition Institute website: http://hni.ilsi.org/ publications. -

Carotene and Novel Apocarotenoid Concentrations in Orange-Fleshed Cucumis Melo Melons: Determinations of Β-Carotene Bioaccessibility and Bioavailability Matthew K
ARTICLE pubs.acs.org/JAFC Carotene and Novel Apocarotenoid Concentrations in Orange-Fleshed Cucumis melo Melons: Determinations of β-Carotene Bioaccessibility and Bioavailability Matthew K. Fleshman,† Gene E. Lester,*,‡ Ken M. Riedl,§ Rachel E. Kopec,§ Sureshbabu Narayanasamy,|| Robert W. Curley, Jr.,|| Steven J. Schwartz,§ and Earl H. Harrison*,† † Department of Human Nutrition, The Ohio State University, Columbus, Ohio 43210, United States ‡ Kika de la Garza Subtropical Agricultural Research Center, United States Department of Agriculture, Weslaco, Texas 78596, United States § Department of Food Science and Technology, The Ohio State University, Columbus, Ohio 43210, United States College) of Pharmacy, The Ohio State University, Columbus, Ohio 43210, United States ABSTRACT: Muskmelons, both cantaloupe (Cucumis melo Reticulatus Group) and orange-fleshed honeydew (C. melo Inodorus Group), a cross between orange-fleshed cantaloupe and green-fleshed honeydew, are excellent sources of β-carotene. Although β- carotene from melon is an important dietary antioxidant and precursor of vitamin A, its bioaccessibility/bioavailability is unknown. We compared β-carotene concentrations from previously frozen orange-fleshed honeydew and cantaloupe melons grown under the same glasshouse conditions, and from freshly harvested field-grown, orange-fleshed honeydew melon to determine β-carotene bioaccessibility/bioavailability, concentrations of novel β-apocarotenals, and chromoplast structure of orange-fleshed honeydew melon. β-Carotene and β-apocarotenal concentrations were determined by HPLC and/or HPLC-MS, β-carotene bioaccessibility/ bioavailability was determined by in vitro digestion and Caco-2 cell uptake, and chromoplast structure was determined by electron microscopy. The average β-carotene concentrations (μg/g dry weight) for the orange-fleshed honeydew and cantaloupe were 242.8 and 176.3 respectively. -

Carotenal, Ethyl Ester of Β-Apo-8’-Carotenoic Acid, Lutein, Zeaxanthin and Concluding Remarks
The EFSA Journal (2009) 1098, 1-48 SCIENTIFIC OPINION Safety of use of colouring agents in animal nutrition1 Part III: β-apo-8’-carotenal, ethyl ester of β-apo-8’-carotenoic acid, lutein, zeaxanthin and concluding remarks Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed (Question No EFSA-Q-2003-060) Adopted on 12 May 2009 ∗ PANEL MEMBERS Georges Bories, Paul Brantom, Joaquim Brufau de Barberà, Andrew Chesson, Pier Sandro Cocconcelli, Bogdan Debski, Noël Dierick, Jürgen Gropp, Ingrid Halle, Christer Hogstrand, Joop de Knecht, Lubomir Leng, Sven Lindgren, Anne-Katrine Lundebye Haldorsen, Alberto Mantovani, Miklós Mézes, Carlo Nebbia, Walter Rambeck, Guido Rychen, Atte von Wright and Pieter Wester SUMMARY Following a request from the European Commission, the European Food Safety Authority (EFSA) was asked to deliver a scientific opinion on the safety of use of colouring agents in animal nutrition. β-apo-8’-carotenal (trans-β-apo-8’-carotenaldehyde, known also as CI Food Orange 6) occurs abundantly in nature, although in very low concentrations. The effective feed concentration to colour egg yolk is 40 mg β-apo-8’-carotenal kg-1 complete feed for laying hens. However, when a red-pigmenting carotenoid like canthaxanthin is additionally used, the same effect will be reached with 10 mg β-apo-8’-carotenal kg-1 complete feed. Data for skin pigmentation are not available. Very limited information is available concerning the metabolic fate of β-apo-8’-carotenal in poultry. Dietary β-apo-8’-carotenal is deposited in the egg yolk as its oxidation product β-apo- 8’-carotenoic acid.