NIH Public Access Author Manuscript Int Ophthalmol Clin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neovascular Glaucoma: Etiology, Diagnosis and Prognosis
Seminars in Ophthalmology, 24, 113–121, 2009 Copyright C Informa Healthcare USA, Inc. ISSN: 0882-0538 print / 1744-5205 online DOI: 10.1080/08820530902800801 Neovascular Glaucoma: Etiology, Diagnosis and Prognosis Tarek A. Shazly Mark A. Latina Department of Ophthalmology, Department of Ophthalmology, Massachusetts Eye and Ear Massachusetts Eye and Ear Infirmary, Boston, MA, USA, and Infirmary, Boston, MA, USA and Department of Ophthalmology, Department of Ophthalmology, Tufts Assiut University Hospital, Assiut, University School of Medicine, Egypt Boston, MA, USA ABSTRACT Neovascular glaucoma (NVG) is a severe form of glaucoma with devastating visual outcome at- tributed to new blood vessels obstructing aqueous humor outflow, usually secondary to widespread posterior segment ischemia. Invasion of the anterior chamber by a fibrovascular membrane ini- tially obstructs aqueous outflow in an open-angle fashion and later contracts to produce secondary synechial angle-closure glaucoma. The full blown picture of NVG is characteristized by iris neovas- cularization, a closed anterior chamber angle, and extremely high intraocular pressure (IOP) with severe ocular pain and usually poor vision. Keywords: neovascular glaucoma; rubeotic glaucoma; neovascularization; retinal ischemia; vascular endothe- lial growth factor (VEGF); proliferative diabetic retinopathy; central retinal vein occlusion For personal use only. INTRODUCTION tive means of reversing well established NVG and pre- venting visual loss in the majority of cases; instead bet- The written -

Fibroblast Growth Factor-2 Drives and Maintains Progressive Corneal Neovascularization Following HSV-1 Infection
ARTICLES Fibroblast growth factor-2 drives and maintains progressive corneal neovascularization following HSV-1 infection HR Gurung1, MM Carr2, K Bryant2, AJ Chucair-Elliott2 and DJJ Carr1,2 Herpes simplex virus type 1 (HSV-1) infection of the cornea induces vascular endothelial growth factor A (VEGF-A)- dependent lymphangiogenesis that continues to develop well beyond the resolution of infection. Inflammatory leukocytes infiltrate the cornea and have been implicated to be essential for corneal neovascularization, an important clinically relevant manifestation of stromal keratitis. Here we report that cornea infiltrating leukocytes including neutrophils and T cells do not have a significant role in corneal neovascularization past virus clearance. Antibody- mediated depletion of these cells did not impact lymphatic or blood vessel genesis. Multiple pro-angiogenic factors including IL-6, angiopoietin-2, hepatocyte growth factor, fibroblast growth factor-2 (FGF-2), VEGF-A, and matrix metalloproteinase-9 were expressed within the cornea following virus clearance. A single bolus of dexamethasone at day 10 post infection (pi) resulted in suppression of blood vessel genesis and regression of lymphatic vessels at day 21 pi compared to control-treated mice. Whereas IL-6 neutralization had a modest impact on hemangiogenesis (days 14–21 pi) and lymphangiogenesis (day 21 pi) in a time-dependent fashion, neutralization of FGF-2 had a more pronounced effect on the suppression of neovascularization (blood and lymphatic vessels) in a time-dependent, leukocyte- independent manner. Furthermore, FGF-2 neutralization suppressed the expression of all pro-angiogenic factors measured and preserved visual acuity. INTRODUCTION corneal neovascularization is topical administration of steroid The cornea is an immune privileged tissue and its transparency is or non-steroid anti-inflammatory drugs, unwarranted side critical for optimal visual acuity. -
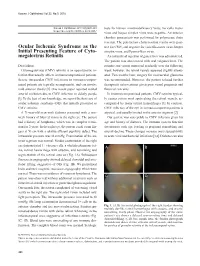
Ocular Ischemic Syndrome As the Initial Presenting Feature of Cyto
Korean J Ophthalmol Vol.32, No.5, 2018 Korean J Ophthalmol 2018;32(5):428-429 tests for human immunodeficiency virus, varicella zoster https://doi.org/10.3341/kjo.2018.0017 virus and herpes simplex virus were negative. An anterior chamber paracentesis was performed for polymerase chain reaction. The polymerase chain reaction results were posi- Ocular Ischemic Syndrome as the tive for CMV, and negative for varicella-zoster virus, herpes Initial Presenting Feature of Cyto- simplex virus, and Epstein-Barr virus. megalovirus Retinitis An intravitreal injection of ganciclovir was administered. The patient was also treated with oral valganciclovir. The Dear Editor, retinitis and vitritis improved gradually over the following Cytomegalovirus (CMV) retinitis is an opportunistic in- week; however, the retinal vessels appeared slightly attenu- fection that usually affects immunocompromised patients. ated. Two months later, surgery for neovascular glaucoma Severe intraocular CMV infections in immunocompro- was recommended. However, the patient refused further mised patients are typically asymptomatic, and can involve therapeutic interventions given poor visual prognosis and mild anterior uveitis [1]. One recent paper reported retinal financial concerns. arterial occlusion due to CMV infection in elderly people In immunocompromised patients, CMV retinitis typical- [1]. To the best of our knowledge, we report the first case of ly causes cotton wool spots along the retinal vessels, ac- ocular ischemic syndrome (OIS) that initially presented as companied by many retinal hemorrhages [1]. In contrast, CMV retinitis. CMV infection of the eye in immunocompetent patients is A 71-year-old man with diabetes presented with a one- atypical, and usually limited to the anterior segment [2]. -

Retinal Emergencies
When to Refer to RETINA Joseph M. Coney, MD February 17, 2017 Memphis, TN Financial Disclosure Commercial Interest What was received For what role Aerpio Grant Support Contracted Research Alcon Laboratories Grant Support Contracted Research Alimera Consulting Fee Consultant/Advisor Allergan Consulting Fee Consultant/Advisor Allergan Grant Support Contracted Research Apellis Grant Support Contracted Research Genentech Grant Support Contracted Research Genentech Consulting Fee Consultant/Advisor Hoffman La Roche Grant Support Contracted Research Lowy Medical Research Institute Grant Support Contracted Research Notal Vision Consulting Fee Consultant/Advisor Ohr Grant Support Contracted Research Ophthotech Grant Support Contracted Research Regeneron Equity Ownership Interest Regeneron Consulting Fee Consultant/Advisor Tyrogenex Grant Support Contracted Research Overview – Alphabet Soup . Endophthalmitis/IOI . VH . CRAO . CRVO/BRVO . PVD . NPDR/PDR . RD . ERM . NVI/NVA . MH . CNVM . VMT Endophthalmitis Hypopyon Hypopyon Bacterial Endophthalmitis . Types . Acute post-operative . Chronic post-operative . Bleb-associated . Endogenous . Intravitreal injection-related . Post-traumatic Bacterial Endophthalmitis . Course/Prognosis . Depends on . type of endophthalmitis . duration of time to presentation & Rx . virulence of organism . Bleb-associated, post-traumatic, endogenous endophthalmitis have poorest prognosis Bacterial Endophthalmitis . Presenting Symptoms . Decreased vision . Pain . Red eye . Swollen lid . Hypopyon . Most common organisms – Acute Post-op . Staphylococcus epidermidis (70%) . Other gram positives (24.2%) . Staph aureus (10%) . Streptococcus (9%) . Enterococcus (2.2%) Bacterial Endophthalmitis . Treatment . Prompt intervention critical to restore vision/salvage globe . Vitreous tap & intravitreal antibiotic injections can be done in office with no delays . In very severe or resistant cases, pars plana vitrectomy in operating room Central Retinal Artery Occlusion CRAO . Incidence . About 1:10,000 general patient visits . Most patients over 60 years . -

Eleventh Edition
SUPPLEMENT TO April 15, 2009 A JOBSON PUBLICATION www.revoptom.com Eleventh Edition Joseph W. Sowka, O.D., FAAO, Dipl. Andrew S. Gurwood, O.D., FAAO, Dipl. Alan G. Kabat, O.D., FAAO Supported by an unrestricted grant from Alcon, Inc. 001_ro0409_handbook 4/2/09 9:42 AM Page 4 TABLE OF CONTENTS Eyelids & Adnexa Conjunctiva & Sclera Cornea Uvea & Glaucoma Viitreous & Retiina Neuro-Ophthalmic Disease Oculosystemic Disease EYELIDS & ADNEXA VITREOUS & RETINA Blow-Out Fracture................................................ 6 Asteroid Hyalosis ................................................33 Acquired Ptosis ................................................... 7 Retinal Arterial Macroaneurysm............................34 Acquired Entropion ............................................. 9 Retinal Emboli.....................................................36 Verruca & Papilloma............................................11 Hypertensive Retinopathy.....................................37 Idiopathic Juxtafoveal Retinal Telangiectasia...........39 CONJUNCTIVA & SCLERA Ocular Ischemic Syndrome...................................40 Scleral Melt ........................................................13 Retinal Artery Occlusion ......................................42 Giant Papillary Conjunctivitis................................14 Conjunctival Lymphoma .......................................15 NEURO-OPHTHALMIC DISEASE Blue Sclera .........................................................17 Dorsal Midbrain Syndrome ..................................45 -

Hyphema. Part I. Pathophysiologic Considerations
University of Pennsylvania ScholarlyCommons Departmental Papers (Vet) School of Veterinary Medicine 11-1-1999 Hyphema. Part I. Pathophysiologic Considerations András M. Komáromy David T. Ramsey Dennis E. Brooks Cynthia C. Ramsey Maria E. Kallberg See next page for additional authors Follow this and additional works at: https://repository.upenn.edu/vet_papers Part of the Ophthalmology Commons, and the Veterinary Medicine Commons Recommended Citation Komáromy, A. M., Ramsey, D. T., Brooks, D. E., Ramsey, C. C., Kallberg, M. E., & Andrew, S. E. (1999). Hyphema. Part I. Pathophysiologic Considerations. Compendium on Continuing Education for the Practicing Veterinarian, 21 (11), 1064-1069. Retrieved from https://repository.upenn.edu/vet_papers/51 Dr. Komáromy was affiliated with the University of Pennsylvania from 2003-2012. Part II can be found at http://repository.upenn.edu/vet_papers/52/ This paper is posted at ScholarlyCommons. https://repository.upenn.edu/vet_papers/51 For more information, please contact [email protected]. Hyphema. Part I. Pathophysiologic Considerations Abstract Hemorrhage in the anterior chamber of the eye, or hyphema, results from a breakdown of the blood-ocular barrier (BOB) and is frequently associated with inflammation of the iris, ciliary body, or retina. Hyphema can also occur by retrograde blood flow into the anterior chamber via the aqueous humor drainage pathways without BOB breakdown. Hyphema attributable to blunt or perforating ocular trauma is more common than that resulting from endogenous causes. When trauma has been eliminated as a possible cause, it is prudent to assume that every animal with hyphema has a serious systemic disease until proven otherwise. Disciplines Medicine and Health Sciences | Ophthalmology | Veterinary Medicine Comments Dr. -

Neuro-Ophthalmological Emergency Disorders: a General View
Open Access International Journal of Clinical and Experimental Ophthalmology Mini Review Neuro-ophthalmological emergency disorders: A general ISSN 2577-140X view Burak Turgut1*, Feyza Çaliş Karanfi l1 and Fatoş Altun Turgut2 1Yuksek Ihtisas University, Faculty of Medicine, Department of Ophthalmology, Ankara, Turkey 2Elazig Training and Research Hospital, Elazig, Turkey *Address for Correspondence: Burak Turgut, Abstract Professor of Ophthalmology Yuksek Ihtisas University, Faculty of Medicine, Department of Neuro-ophthalmological emergency disorders usually occur with symptoms of visual loss, diplopia, ocular Ophthalmology 06520, Ankara, Turkey, Tel: +90 motility impairment and anisocoria. In this mini-review, we aim to take look the common neuro-ophthalmological 312 2803601; Fax: +90 3122803605; Email: emergency disorders. The delayed diagnosis of the neuro-ophthalmological emergencies puts the patient at [email protected] risk of death or blindness. If these are well-known, the discrimination and management of these emergency Submitted: 18 December 2017 conditions will be easier. Approved: 26 December 2017 Published: 27 December 2017 Copyright: 2017 Turgut B, et al. This is Introduction an open access article distributed under the Creative Commons Attribution License, which Neuro-ophthalmology is a subspecialty of both neurology and ophthalmology permits unrestricted use, distribution, and training the visual pathways including the optic nerve, chiasm, optic tract, lateral reproduction in any medium, provided the geniculate nucleus, -

Ocular Ischemic Syndrome During Carotid Balloon Occlusion Testing
Ocular Ischemic Syndrome during Carotid Balloon Occlusion Testing Eric J. Russell, Kenneth Goldberg, James Oskin, Crystal Darling, and Onur Melen Summary: The use of a double-lumen balloon catheter for The use of a double-lumen balloon catheter for temporary occlusion testing of the internal carotid artery per temporary occlusion of the carotid artery permits mits simultaneous perfusion of the distal internal carotid artery simultaneous perfusion of the artery (with hepa (and ophthalmic artery) with heparinized saline. If saline is rinized saline) beyond the obstructing balloon. infused too rapidly, the result may be total or partial replace This helps to minimize the risk of distal throm ment of oxygenated blood within the ophthalmic artery. This replacement may produce the signs and symptoms of ocular boembolism, but saline replaces blood within the ischemic syndrome. These include ipsilateral orbital pain and carotid artery beyond the balloon. Too rapid an progressive uniocular visual loss. Simple technical adjustments infusion of saline, administered by a pressure In the performance of the occlusion test can prevent the devel pack, may result in total or partial replacement opment of this syndrome. Failure to recognize the cause of the of oxygenated blood within the ophthalmic artery observed visual loss may produce the false impression of a (Fig 1). This replacement produces the signs and positive occlusion test or may falsely suggest that a throm symptoms of ocular ischemic syndrome, includ boembolic complication has occurred. Awareness of the occur ing ipsilateral orbital pain and associated progres rence of this syndrome should prevent confusion concerning sive visual loss. the predictive result of provocative carotid occlusion testing. -

Observation of Optic Disc Neovascularization Using OCT
www.nature.com/scientificreports OPEN Observation of optic disc neovascularization using OCT angiography in proliferative Received: 13 December 2017 Accepted: 16 February 2018 diabetic retinopathy after Published: xx xx xxxx intravitreal conbercept injections Xiao Zhang1, Chan Wu1, Li-jia Zhou2 & Rong-ping Dai1 This study reports the short-term efcacy and safety of intravitreal conbercept injections for neovascularization at the disc (NVD) in patients with proliferative diabetic retinopathy (PDR). Conbercept is a recombinant fusion protein with a high afnity for all isoforms of vascular endothelial growth factor (VEGF)-A, placental growth factor and VEGF-B. A prospective case series study was conducted in 15 patients (15 eyes). Patients had complete ocular examinations and received a 0.5 mg intravitreal conbercept injection followed by supplemental pan-retinal photocoagulation (PRP). Optical coherence tomography angiography (OCTA) was performed before and after treatment. Before treatment, the mean NVD area was 1.05 ± 0.33 mm2, and it decreased to 0.56 ± 0.17 mm2 after an interval of 7.5 d (p = 0.000). One eye required vitrectomy during follow-up. Recurrent NVD was observed in 2 eyes, which resolved after repeated injections. The remaining 12 eyes were stable over a mean follow-up period of 8.3 months. The mean area of the NVD in 14 patients without vitrectomy was 0.22 ± 0.11 mm2 (p = 0.000) at the last visit. Intravitreal conbercept injections combined with intensive PRP are an efective and safe treatment for PDR with NVD. Quantitative information on NVD can be obtained with OCTA, which may be clinically useful in evaluating the therapeutic efect. -

Clinical Applications of Non-Invasive Multimodality Imaging in The
The Egyptian Journal of Hospital Medicine (April 2021) Vol. 82 (3), Page 974-981 Clinical Applications of Non-Invasive Multimodality Imaging in The Evaluation of Ocular Ischemic Syndrome: A Case Study Abdulhamid Alghamdi Faculty of Medicine, Department of Ophthalmology, Taif University Email: [email protected]; Tel: +99 733 44 88 Fax: +99 733 4567 ABSTRACT Background: A 74 years old lady presented with bilateral sudden deterioration of vision over a period of 3 weeks. She is a known diabetic and hypertensive for the past 25 years. The patient denied associated neurological symptoms. The patient underwent a comprehensive non-invasive neuroimaging and retinal imaging. Case Presentation: The case study is about right sided occipital lobe infarction and right sided internal carotid artery stenosis with complete right ophthalmic artery occlusion resulted in right Ocular ischemic syndrome (OIS). Ocular findings showed normal swinging light reflex. Right eye showed rubiosis iridis, neovascularization of the disc. Left eye showed moderate non-proliferative diabetic retinopathy. There was lack of cerebellar and/or cortical constitutional symptoms. Magnetic resonance angiography (MRA) imaging showed compromised right side internal carotid system and complete occlusion of the right ophthalmic artery. Magnetic resonance imaging (MRI) showed as well isolated right sided occipital lobe infarction. The patient received neurological and ocular treatment. Conclusion: Painless sudden bilateral visual disturbance in an elderly individual should draw the clinician attention to possible cortical etiology inspite the absence of neurological symptoms. Non-invasive imaging techniques had been shown to be effective in the evaluation of symptomatic Ocular ischemic syndrome (OIS), which should be considered if the gold standards imaging techniques are relatively or absolutely contraindicated. -

Iris Rubeosis, Severe Respiratory Failure and Retinopathy of Prematurity – Case Report
View metadata, citation and similar papers at core.ac.uk brought to you by CORE +MRIOSP4SPprovided by Via Medica Journals 46%')/%>9-78='>2) neonatologia Iris rubeosis, severe respiratory failure and retinopathy of prematurity – case report Rubeoza tęczówki, ciężka niewydolność oddechowa i retinopatia wcześniaków – opis przypadku 0RQLND0RGU]HMHZVND8UV]XOD.XOLN:RMFLHFK/XELĔVNL Department of Ophthalmology, Pomeranian Medical University, Szczecin, Poland Abstract The aim: Case study reports for the first time about development of massive iris neovascular complication in co- urse of retinopathy of prematurity related to systemic and ocular ischemic syndrome due to tracheostomy-requiring extremely severe premature respiratory failure. Material and method: Premature female, 950 grams birth weight, born from 17-year-old gravida 1, at 28 weeks’ gestation by cesarean section due to premature placental abruption with threatening hemorrhages, with 1 to 5 Apgar score. The baby developed severe respiratory failure which required tracheostomy, advanced bronchopulmo- nary dysplasia treated with steroids (BPD) and respiratory distress syndrome (RDS) with failure to extubate together with secondary ocular ischemia. All the mentioned with multifactorial organs complications (NEC, leucopenia, ane- mia, pneumonia, periventricular leucomalacia, electrolyte abnormalities and metabolic acidosis) resulted in massive peripupillary iris neovascularization (NVI) in both eyes coexisting with retinopathy of prematurity (ROP) in 38 weeks’ PMA infant. Ultrasonography-B, slit-lamp and indirect fundus examinations with photography were used to document focusing ocular diagnosis. The previous retinopathy of prematurity screening examinations performed at standard intervals of time starting from four weeks of life, that is 32 weeks’ PMA continuing every two weeks did not present typical lesions seen in retinopathy, however in the second zone of retina slightly marked “plus sign” was visible. -

Case Report of Ocular Ischemic Syndrome, Taif, Saudi Arabia
The Egyptian Journal of Hospital Medicine (January 2018) Vol. 70 (9), Page 1710-1713 Case Report of Ocular Ischemic Syndrome, Taif, Saudi Arabia Abdulmohsen Hamad Hamed Alhamyani1 , Sami Awd Alharthi, Sara Hussein A Gebril2 , Mohamed Ahmed Siddig,Osama A Alhaj , Nuha Mohamed Ahmed2, Talal Abdulrahman Althomali3 Taif medical college1, King Faisal hospital2, Taif university3 ,Taif ,Saudi Arabia ABSTRACT Background: Ocular ischemic syndrome (OIS) is more prevalent among male aged more than 50 years. Various disorders such as diabetes, hypertension, and peripheral vascular diseases are major risk factors responsible for OIS in aged male. Case report: This case study involves a 57-year-old male patient having history of diabetes, hypertension, slurred speech, hemiparesis and hypoesthesia of the right side of the body. The patient came with complain of red eye, pain and decreased vision in left eye. Complete left internal carotid artery obstruction, rubeosis iridis of the left eye, dots of hyphema of the left eye, bilateral hard exudates, significant macular edema and non-proliferative diabetic retinopathy were observed in the patient. In this case, in consultation with ophthalmology, surgery and medicine departments multidrug treatment procedure was followed. This multi- drug based therapy successfully controlled the condition of patient and improved the vision of patient. Keywords: Ocular ischemic syndrome, left internal carotid obstruction. INTRODUCTION prothrombin time 13.7 second were also Ocular ischemic syndrome (OIS ) affects vision determined to get comprehensive view of clinical and if not timely treated it may cause vision loss status of the patient. Complete blood count is [1]. Diabetes, stroke, cardiac vascular diseases, shown in table (1).